An excellent new study from Thailand just got published on May 30th in Frontiers in Cell and Developmental Biology. It examines the current status and advances in hair regeneration via induced pluripotent stem cells (iPSCs).
It also compares in detail hair regeneration via this method versus via follicular cell sources. I found this bifurcation very interesting.
I covered Thailand in past posts regarding doctors over there pioneering the use of oral Minoxidil for hair growth (20 plus years ago). Note that Thailand is also the world’s leader when it comes to gender reassignment surgery.
Induced Pluripotent Stem Cells for Hair Regeneration
The two authors of this study are from the Siriraj Center for Regenerative Medicine and the faculty of medicine at Siriraj Hospital.
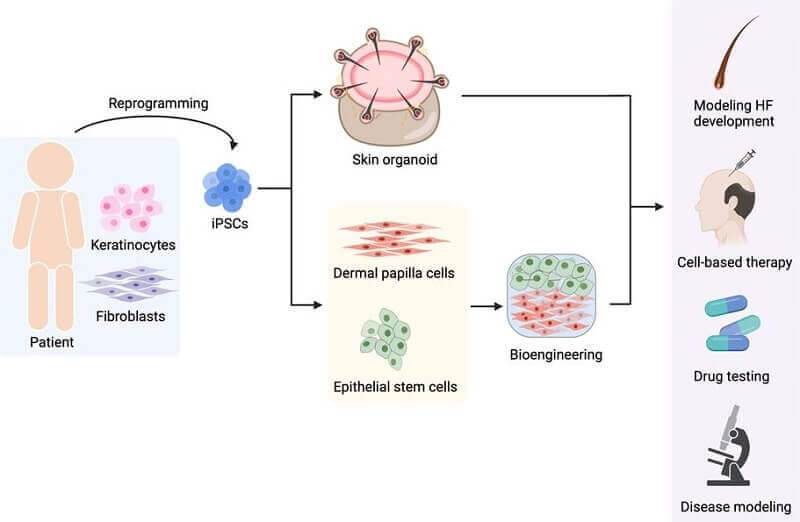
When it comes to hair follicle regeneration from human induced pluripotent stem cells (hiPSCs), the authors break it out into the following categories:
- Generation of individual hair follicle components. Further broken out into a) iPSC-derived trichogenic dermal cells. b) iPSC-derived folliculogenic epidermal cells. c) In vitro reconstruction of HFs with iPSC-derived dermal and epidermal cells.
- Generation of entire hair follicles from iPSCs. Further broken out into a) 3D integumentary organ system (IOS). b) Skin organoid.
When it comes to the 3D IOS method, they give the example of Dr. Takashi Tsuji and his RIKEN team’s success at generating a bioengineered 3D integumentary organ system (IOS) from mouse iPSCs (miPSCs). They also refer to studies by Ohyama and Tsuboi (lead researcher at Shiseido) that I have discussed in the past.
They also mention Dr. Koehler’s work that I covered in my post on hair-bearing human skin generated entirely from pluripotent stem cells. And of course they mention Stemson Therapeutics co-founder Dr. Terskikh and his past papers. When I interviewed the latter in 2017, he mentioned iPSCs in great detail.
Other subjects covered in the paper that I discussed in the past include biomimetic engineering of human hair and 3D culturing of hair cells.
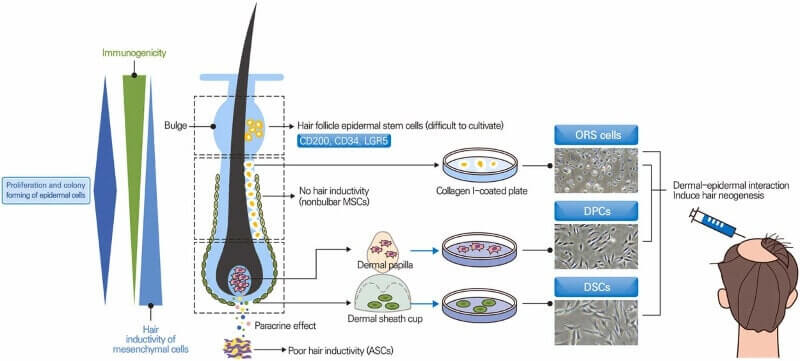
Hair Follicle Regeneration from Follicular Cell Sources
The authors divide the follicular cell based hair regeneration methods into the following categories:
- Dermal papilla cells. Several decades ago, Aderans and Intercytex both saw some success in hair growth via dermal papilla cell culturing and injection into balding scalps. HairClone is currently trying something similar. And South Korea’s Epibiotech and Han Bio both seem to be rapidly progressing with this technology.
- Dermal sheath cup cells. This is what Shiseido is doing in Japan via the use and improvement of Replicel (Canada)’s technology.
- Hair follicle stem cells. The main disadvantage of this method with current culturing methods is rapid loss of stem cell abilities and spontaneous differentiation. Of interest, they mention a new March 2023 study from Fukuda et. al in relation to hair follicle stem cell expansion in hair regenerative medicine.
Make sure to also read my November 2022 post on effective cell therapy for hair regeneration. It was based on a very detailed new research paper authored by Epibiotech’s CEO.
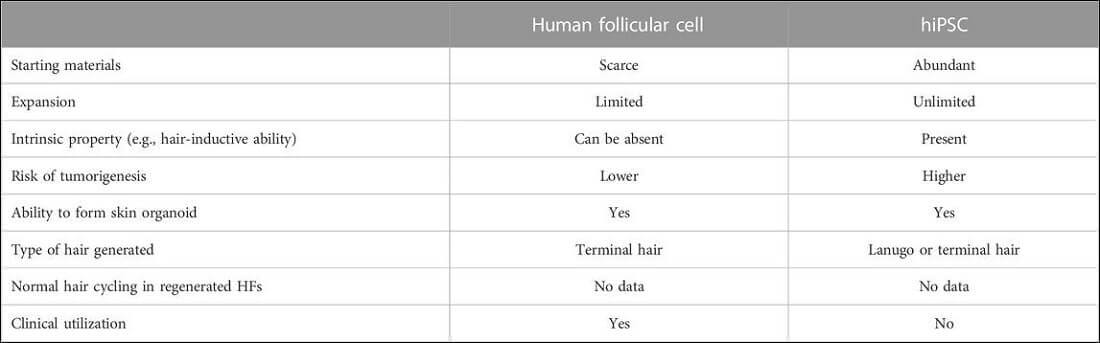
Comparison of Cell Based vs hiPSC Based Methods
The below table from the new study is very useful. It compares the advantages and disadvantages of each method of hair regeneration.
The clear danger of the hiPSC method is potential tumorigenesis. Hence the reason why Stemson Therapeutics and OrganTech have to go through rigorous clinical trials. However, in return, iPSCs have the advantages of unlimited starting material, unlimited expansion and intrinsic hair-inductive ability.
Conclusion
The paper makes the following statements towards the end that are of significance:
- Autologous transplantation of dermal sheath cup cells (DSCs) is useful for patients with male and female pattern hair loss.
- The generation of DSCs from hiPSCs may provide an unlimited source of cells for transplantation.
- Nevertheless, bioengineered hair follicles are still required for some type of hair loss that involve entire hair follicles. Therefore, generating hair follicles through a biomimetic developmental approach is of interest.
- Recent understanding of hair biology and iPSC technology offers hope for the generation of hair follicle components and entire hair follicles from hiPSCs.
- Several approaches for reconstructing hair follicles from hiPSCs have been established. However, fully functional bioengineered hair follicles have yet to be developed.
- Nevertheless, the authors conclude that these newer strategies for de novo folliculogenesis bring us one step closer to the ultimate goal.