In 2004, Aderans Research Institute filed a patent (granted in 2009) titled “Tissue engineered biomimetic hair follicle graft“. The invention entailed an improved scaffold that would mimic the architecture of the native hair follicle. The ultimate aim for this invention after further improvements would be hair multiplication. However, for a number of reasons, the much hyped Aderans liquidated its research institute in 2013.
In the above patent filing, the most cited author when it comes to reference material was Durham University (UK)’s Dr. Colin Jahoda. To be specific, 10 of his past papers are cited: ranging from this one from 1981 to a 2001 paper on trans-species hair growth induction. The industrious Dr. Jahoda has published numerous other major research papers since 2001, some of which I have covered on this blog in the past.
Biomimetic Engineering of Human Hair
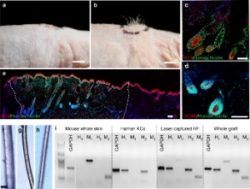
Several days ago, a groundbreaking new research paper was published in Nature Communications. The title of this paper was: “Tissue engineering of human hair follicles using a biomimetic developmental approach”. Very similar to the title of the earlier mentioned patent.
Moreover, one of the main co-authors of this latest 2018 work is Durham University’s Dr. Colin Jahoda. The other authors are all researchers from US-based Columbia University’s Department of Dermatology, led by the renowned Dr. Angela Christiano.
The conclusion of this research is one that should make everyone enthused:
“The ability to regenerate an entire hair follicle from cultured human cells will have a transformative impact on the medical management of different types of alopecia, as well as chronic wounds, which represent major unmet medical needs.”
Note that this latest paper was submitted in May 2018, accepted in October 2018, and finally published in December 2018. So the Jahoda, Christiano et al. team’s current research is at least seven months ahead of what is described in the paper.
3D-Spheroid Cultures to 3D-Printed Molds
I have covered 3D-spheroids, 3D-culturing and related structures and scaffolds (to help brand new hair follicles grow from scratch) numerous times. This area of research has seemed to be the holy grail for scientists trying to succeed at hair multiplication. Just like DHT elimination and restarting Wnt/β-catenin signaling have been the holy grails when it comes to preventing further hair loss and regrowing existing miniaturized hair.
Numerous scientists such as Dr. Colin Jahoda and Dr. Takashi Tsuji have focused on research 3D-spheroids and 3D-culturing of dermal papilla cells to grow new hair follicles for many years. However, in this latest study, it seems like the scientists have turned there focus to 3D-printing (or 3D-bioprinting). They even give the name of the specific 3D printer that they used during this experimentation.
The Jahoda, Christiano et. al team created 3D-printed hair follicle molds as the key component of the experiment. The scientists used a biomimetic approach to generate human hair follicles within human skin constructs (HSCs). They emulated human biology via the 3D organization of cells in the hair follicle micro-environment using 3D-printed molds. The actual paper is very technical.
The authors suggest that in the future, 3D bioprinting technology operating at a single cell resolution may permit the inclusion of many other cell types. This would include stem cells and melanocytes, which would generate hair cycling and pigmented hair follicles.
Some interesting quotes from the paper:
“We recently addressed this issue by 3D-spheroid culture of cells and thereby restored 22% of the hair inductive DPC gene signature. Subsequently, other groups also reported the use of this method to induce HFs in mice, albeit inefficiently. To enhance the efficiency of hair induction properties, in this study, we combined genetic and microenvironmental reprogramming strategies by overexpressing the MR gene Lef-1 in combination with spontaneous DPC spheroid formation in the HSCs, which resulted in 70% success rate of HF formation ex vivo, compared to only 19% with the empty vector-transfected DPCs.”
“Using 3D-printing approaches, our goal is to engineer HFs as follicular units and/or in desired patterns that can be integrated with surgical robots and facilitate effective hair transplantation surgery.”
Please apply this biometric engendering system on human melanocytes in order to treat Vetilligo specially the non segmental wide spreading type . The 3D biometric technology will allow the cultured melanocyte cells to be reprogrammed as resistant cells to the autoimmune factors then recultured by meme robots to the skin to provide a victory over on of the most difficult problems in Dermatologic therapy .
http://www.xinhuanet.com/english/2018-12/07/c_137657212.htm
just for all who is interested, replicel is trading at 32 CAD cents at the min, i now have 4000 shares :). i know this is probably in the wrong section but sure
Its at .24 now ur at -300 something. But I hope eventually stock starts trending up and you make a healthy return. Also when is Shiseido supposed to announce the results of the trial?
So it all sounds good so when can we see it in reality?
I mean, Takashi Tsuji is doing the same thing in Japan. As far as I’m aware, he will be the first to do clinical tests on humans in early 2019. So probably in a couple of years, if it works.
I’m pretty optimistic that it will work though. You see that multiple universities and researchers are confirming findings. This means that we are truly on to something, if Tsuji is succesful, things can move pretty quick. (but that’s a big if, since, as mentioned in the paper, there still exists a lot of difference between the hair follicles of a mouse and a human (but it’s obviously not that remote)).
So, if you look at the history of science, you pretty much see just that. Not just one person having a breakthrough, but multiple scientists kind of reaching the same conclusions, but one get’s slightly ahead of the others and is/are deemed the ‘inventor(s)’ of said invention.
Another aspect of this approach is that we don’t really need to know ‘why’ people bald, we can just clone and fix it. Obviously, future research can figure out a why and find a more effective treatment. But being able to ‘cure baldness’ without knowing what causes baldness, do you care?
i think the why still matters but having a functional cure would definitely alleviate urgency for the a more effective treatment..
My guess would be it’s genetic and something like crispr would be able to reverse it.
This does sound pretty exciting. If they would’ve announced a company name it would have been better.
Very cool stuff. Along these lines, I’m hoping we see some trial results from Riken next year!
Hi all,
Apologies for the off topic. Do you if Trinov is already available in Italy? I am asking because I could not find it on google and I did read it should have been released early December.
(FYI, I am not asking for the quality of the product or if it works or not. Just asking the if it is purchasable)
According to Tuttopharma, sounds like sales in Italy are off to a brisk start as “shipments are scheduled after January 12th, due to stock exhaustion by the manufacturer.” No sales yet outside of Italy as far as I can tell.
As a further update on timing, it does not appear it is available yet outside of Italy and even if it were, it appears that customers are limited to 1 bottle per order because of the mismatch in demand vs production. So if you are planning on ordering from the US, it would be very expensive ($30+ per shipment) since you can’t yet buy in bulk. No word on whether they can ramp up production in the near term.
A novel and safe small molecule enhances hair follicle regeneration by facilitating metabolic reprogramming.
https://www.ncbi.nlm.nih.gov/pubmed/30523246
Furthermore, the stem cell population with a glycolytic metabotype appeared slightly earlier in the IM-treated mice. Stem cell and niche signaling involved in the hair regeneration process was also activated by the IM treatment during the early phase of hair follicle regeneration. Overall, these results show that the novel small molecule IM promotes tissue regeneration, specifically in hair regrowth, by restructuring the metabolic configuration of stem cells.
Okay, so they’re saying they have a 70% success rate with producing functional hair follicles outside the body? (with some type of 3D printer spheroid combo)
Is that the plain language of it?? Someone please verify. If so, that’s phenomenal!
It is. Basically, 2008 some guy found out you could reprogram cells. You can make HF’s from that fact. It’s the following finding: https://en.wikipedia.org/wiki/Induced_pluripotent_stem_cell
Thanks M. Yeah, efficiently making hair follicles outside the human body is the holy grail. There shouldn’t be any side effects from that; it’s just like a basic hair transplant with unlimited donor. It’s amazing that multiple research groups are claiming this ability now! Once we see some human patient photos, it won’t be long to get to market.
cool .. hope tsuji gets it to us first.
Slick- what makes you think this will be out in the market so soon? Doesn’t it have to complete all three phases of fda trials? Am I missing something here? It seems like they just started this in mice. Follica has been in trials since 2008 and they are just inventing a new derma stamp device with minox and already approved drugs. Imagine how long it will take for newly stem cell created follicles to be allowed to be implanted and used for Comercial use. Just being realistic here.
You always repeat the same stuff. This is autologous (your own hair) HM and safe and if you did not realize it after all these years here, you must be retarded.
MJones, I said that once we see pictures of it in humans, it won’t be long… Once we see pictures in humans, it’ll be far along in trials, and they’ll rush that huge proven cash cow to market. And finally, Riken has the same basic tech, and they’re claiming a 2020 release in Japan. If we see pictures of hair growth in humans in 2019, something will go to market very soon after. I’m really hoping that Riken’s current claims and promises are not BS. At some point, at least one company has to be legit. I’m hoping it’s them!
What you’re missing is that we live in a globalized world now, and it doesn’t really matter where in the world the trials get done, for the most part. Japan now has a fast-track system for cell therapy human trials, and if it’s proven and approved in Japan, it’ll become available in Japan and a few other smaller countries that have agreements with Japan to share medical technology. That will create an overwhelming global consensus that the procedure is safe and effective, and even if it’s not available in the US immediately, there will be huge pressure on the FDA to approve it much sooner than they otherwise would have. So even if you don’t want to spend the money to go to Japan to get it, there would probably not be so much of a lag between Japan’s approval and US approval.
Hi, I don’t know if anyone saw this, but it was published in Nature this month. Pretty interesting paper
https://www.nature.com/articles/s12276-018-0185-z
Jray- thanks for calling me a retard during the holidays. I really appreciate that.
You think the fda cares it’s autologous? This will go through lengthy trials 100%. They are not just going to approve it because it was in a research paper and autologous. I know that after all these years of reading this stuff is that they all go through trials. Even kerastem…those are your own cells to…
That’s the same one that “omg” posted, above.
Mjones, you asked questions about “which” study recently when you knew it was a study in the post and also in the comment. I see you do that before too. I bet you have never read even 1 study completely that has been posted here. Guaranteed. Your comments indicate zero knowledge and complete uneducated drama queen.
Jray. You are right I am retarded and a drama queen. Not even being sarcastic. I don’t read the studies. I just glimpse to see if there a pictures or anything that stands out. Either way 10000000% this will need fda trials. So stop being naive and face the fact that this won’t come out in a year or two. Let’s say hypothetically you are right. That still need to test these new my created cloned hair follicles to see if they grow and cycle like normal hair. Plus see if they don’t shrink due to dht. Hair cycles every 3 to years. So at a minimum they will test this 5 years or more to see if it works. They are not going to start cloning hairs next year to thousands of people without checking for growth cycles, hair direction etc. You keep reading those articles and keep trashing me. In the end this is still going to get fda tested.
Exiting discovery for sure and it’s a huge step forward but we still got a long way to go.
Admin sorry if I am being retarded or a drama queen:) I just don’t want new hair loss sufferers thinking this is coming out soon and they forget about current available treatments that could stop their hair loss for 5 to 10 years like Propecia or mimox.
Seems they had to genetically modify the cells to make it work. Very early stage… I wish it was not the case but this is far behind what all of us would wish
I know this post will be going on many different tangents, but that’s just in my nature.
I was just reflecting and noticed that the cure was coming five years ago, since 2001! These “scientist” need to figure out how to cure baldness before 2021. For goodness sakes Admin is closer to the cure then some of the bozos who claim their title as scientists. I’ll try and stay optimistic because I have no other real choice.
On a side note, I just wish that damn HIMs commercial would stop saying baldness is a choice. My wife is now saying I need to order that garbage. It’s just rebranded propecia and garbage minoxidil.
Anyways ladies and gents, we can all look at the mirror and watch as our hair lays in our sink and falls from our bald chrome domes.
Be safe and have a wonderful new year. Another year closer to the cure! Five years to go starting now….!
(Admin: Keep doing what your doing. You have the best centralized site for hair loss information.)
Thanks Ben. Hate those Hims ads too.
Lets hope Tsuji and several others give us some great news in 2019:-)
Admin,is there any way you are willing to contact Dr.Tsuji to ask him about the pre-clinical trail?
Happy holidays Ben! I dislike Hims BS as well…dont we wish it were a really a choice but we know that most the case for many many people. Stay strong brother, we have no other choice! And tell the wifey to relax and educate herself on the ingredients of Hims products before she opens her mouth ha.