Note that prior to its hair multiplication work, Shiseido was already well known in Asia via its Shiseido Adenovital Adenosine based products.
Update: February 8, 2021
RepliCel just came out with a new detailed corporate update. It warns Shiseido to adhere to their mutual agreement. i.e., deliver the full recently completed Phase 2 clinical study data set of RCH-01 in Japan. Shiseido calls the same product S-DSC (dermal sheath cup cells).
Interestingly, Replicel is in the near future also planning to start its own Phase 2 clinical trials for the hair growth product (outside of Asia, where Shiseido has the rights). Probably due to Replicel’s recent success in getting new funding via a partnership with MainPointe Pharmaceuticals.
Update: October 14, 2020
Some rare good news this year. Shiseido just started its second multi-center clinical study on the efficacy and safety of extensive repeated injections of cultured human autologous hair follicle dermal sheath cup cells. The treatment will be tested on 36 male and female pattern hair loss patients. Interestingly, they do not mention Replicel’s name anywhere, indicating continuing unresolved partnership issues.
However, Replicel’s latest Tweet (h/t “Paul”) does not hold back:
Shiseido has quietly launched its second multi-center clinical study of RepliCel’s RCH-01 for the treatment of hair loss in Japan.
Shiseido Clinical Trial Update
Update: March 27, 2020
Shiseido’s latest trial results are finally getting global publicity. Yesterday, lead researcher Dr. Ryoji Tsuboi was interviewed by the Asahi Shimbun. The title of the article clearly states that this technology entails transplantation of ones own cells. Not surprisingly, The Daily Mail has even more details.
Shiseido’s method involves autologous transplantation of dermal sheath cup cells. I am very optimistic that Japan’s favorable regulations will lead to this technology reaching the market in the next several years.
Dr. Tsuboi:
“The result of our study was very encouraging, We were able to show that the study could help develop a new treatment for hair loss.”
Update: March 6, 2020
Update: Fuji Maru sent me the following Japanese press release from Shiseido. The company plans to conduct further clinical trials that will administer DSC cells in multiple areas of the scalp.
Autologous Cell-Based Therapy Success
Reader “John Doe” just discovered this new study from Shiseido. As always, I am assuming that Shiseido uses both Replicel’s technology as well as its own-in house technology. Lead author is Dr. Ryoji Tsuboi from Tokyo Medical University. Toho University Ohashi Medical Center was also involved in the trial.
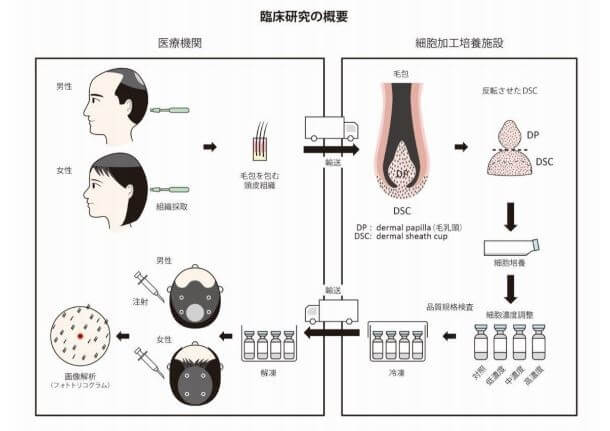
The treatment entails autologous cell–based therapy using dermal sheath cup (DSC) cells, which surround the dermal papilla (DP). Cell manufacturing was conducted at the Shiseido Cell-Processing and Expansion Center (SPEC) and Incubation Center. Both male pattern hair loss and female pattern hair loss patients were treated. 50 males and 15 females, all between the ages of 33-64.
The positive: Total hair density and cumulative hair diameter at injection site significantly increased at 6-months and at 9-months. They seem to imply that this was true for all patients, but maybe someone can access the full study and let us know. Side effects were limited to none in all participating volunteers.
The negative (possibly): The “positive effect was temporary until 9 months”. This statement does not make linguistical sense, and is likely a translation issue. Are they actually trying to say that hair growth was temporary for all of the first 9 months after injection?
Update: Thanks to reader “Left4Bald” for sending me the whole study. They state that the best results were at 6- and 9-months post injection. 12 months had “reduced hair growth”, which is still unclear wording. Further data in the full study indicates that there was a very slight average decline in hair count from 9 to 12 months. Interestingly, older men and women saw best results per phototrichogram before and after images.
Also of significance, 3.0×105 DSC cells was the lowest dose tested. It still elicited a significant increase in total hair density and cumulative hair diameter compared with the placebo. Higher doses did not translate to superior hair growth. The authors suggest that this could be due to an overload of cell debris and immune reactions. In effect causing a poorer environment for the remaining viable DSC cells.
In any case, we should prepare for an eventuality where we need once-a-year DSC injections. Hopefully, Shiseido succeeds in rapidly commercializing this autologous hair growth treatment in regulation friendly Japan.
Update: November 26, 2019
Shiseido Japanese Patent Published
Someone named “Paul” (thanks!) just posted a link to Shiseido’s official Japanese patent, which was released in Japan in May 2019. Paul found this link on an investing forum, and he also posted a bunch of information about stock prices. I deleted the latter, as I do not want to encourage blog readers to buy or sell Shiseido or Replicel stock.
Ultimately, whether “Shiseido decides to do so OR not to do so” will decide everything. The patent indicates optimism about the technology. However, nothing is set in stone for sure. The English translation of the patent is extremely informative if you expand each section and wait for it to load. Or you can open the whole Japanese pdf too and use translation software.
The big question I have is what portion of the technology comes from Replicel (licensing), versus from Shiseido in-house?
The inventors are listed as:
- Tsutomu Soma
- Jiro Kishimoto
- Sayaka Koide
- Hitoshi Okochi
- Masahiro Kiso
Update: November 26, 2019
Replicel Year-End 2019 Update
RepliCel released its 2019 year-end update today. It includes discussion about its partnership with Shiseido. Regarding Replicel’s cellular therapy (RCH-01) for androgenetic alopecia, the key points are that:
- Shiseido’s clinical study in Japan is now complete.
- Shiseido will soon announce on whether it will commercially launch the product in Japan or conduct further clinical testing.
- Replicel is holding off on Shiseido’s final decision before pursuing any further Phase 2 trials. The former’s Phase 1 trials in Europe were successful.
- The Replicel and Shiseido dispute is still unresolved.
It seems like Shiseido could still launch the product in Japan soon. No doubt aided by the country’s new faster regulations for marketing when it comes to cellular therapy.
Replicel’s proprietary injection device, RCI-02, is on schedule to get approval and be used in much of the developed world in 2020.
Replicel-Shiseido Partnership
March 23, 2019
I used to cover Replicel (Canada) and its Japanese cosmetics behemoth partner Shiseido at least a few times per year until last year. At some point, I got skeptical about the RCH-01 autologous cell therapy hair loss product being released any time in the near future.
![]() Besides typical delays, Replicel and Shiseido had some unclear conflicts regarding their partnership.
Besides typical delays, Replicel and Shiseido had some unclear conflicts regarding their partnership.
However, Replicel published two important updates this month, the second of which is of most interest to us:
Per the first link above, the disagreement regarding the agreement between Shiseido and Replicel remains unresolved. However, it is not subject to any litigation or arbitration at this time.
The key money quote is from the second link:
“While the Company’s RCH-01 product for hair loss due to androgenic alopecia may be launched in Japan much earlier if Shiseido decides to do so, current planning anticipates the potential for all four products to be on the market in Japan by 2022.”
The implication seems to be that:
- Shiseido could release the product well before 2022. See my past post on the company’s new research facility in Kobe, Japan.
- Even if Shiseido does not release the product earlier, it will quite possibly get released in 2022 in Japan by Replicel.
See my past post on Japan’s new laws fast-tracking stem cell therapies and clinical trials.
Another key quote:
“Unlike anywhere else in the world, one well-designed cell therapy trial in Japan, approved by their regulatory authorities, has the potential to lead to product market launch.”
Replicel is clearly focusing on a “First-in-Japan” strategy due the country’s favorable regulatory environment. Shiseido has rights to the Asian market when it comes to RCH-01. However, it seems like Replicel can still release the product in Japan in 2022 if Shiseido does not do so?
Hard to make this conclusion for certain without knowing much more about the two companies’ legal contract. In any case, this is a great development.
Further references:
- A discussion of Replicel’s technology in two videos.
Interesting, but if Shiseido chooses not to release it prior to Replicel (2022) wouldn’t one assume it’s not very effective ?
If it’s a home run or even a significant improvement over what we have now , Shiseido would make a killing and would want to cash in right away prior to other companies in the industry also working on similar solutions .
Something doesn’t add up !
Shiseido is a cosmetic company. They’re in the business of selling something you need to use again and again every day. Replicel is marketing an injection process that improves your hair, not something you can do at home indefinitely for a moderate cost. That seems to be the disconnect. Shiseido wanted to see if they could somehow turn the injections into an every-day topical and just couldn’t get the same results and are therefore now less enthused. It doesn’t match their business plan. Hopefully Shiseido can release the injection plan at some point.
Where did you you get your information that Shiseido was trying to turn this into an everyday topical? I tend to agree with Stu. Their premise has been based on culturing cells and no group has figured that out yet, let alone an under-capitalized company like Replicel. I also recall some time ago where a researcher, who was involved in cell culturing, was posting on a site saying that while there had been some success with culturing dermal papillae cells (up to 60% inductivity), there was zero success in culturing dermal sheath cup cells, which is what Replicel is based on. Maybe this has changed, but if you look at Replicel’s stock it is at an all-time low of under 20 cents per share. So if they have had any behind the scenes success, they are very tight lipped about it.
One important side note left out in this article is we know for sure shiseido started human trials over a year and a few months ago.
That being said despite our shaky speculation we know for absolute certain that there are people deep in that company that know for sure whether it works or not right now .. Today.
My only wonder is Why they havent gone public. If it worked wouldnt they already have organized preliminary treatment centers. It makes me doubt success.
The only way it does work is if they are in the marketing process right now. But they said 2018 so….. If they stalled on that release they either scrapped it or tweaking the delivery for a higher outcome. Or it doesnt work at all.
Funny side note not even replicel knows whether it works or not.
Incuded in Replicel’s agreement with Shiseido was a commitment to complete their own Phase 2 trials of RCH-01 in Germany.
When Replicel failed to conduct these trials, Shiseido claimed the terms of their agreement had been broken – and as such Shiseido would no longer share either the results of their trials, or any future revenues, with Replicel.
Replicel disputed this by saying the agreement didn’t stipulate “when” they would conduct the German trials, just that they would.
This is why Replicel now essentially doesn’t know more than anyone else about where Shiseido is at.
In the meantime…..Replicel has been involved in a joint research project with the University of British Columbia seeking to clarify what differences existed in the hair follicle gene expression of subjects who had a strong hair growth response in their earlier RCH-01 phase I/IIa clinical trials and the subjects who had a reduced hair growth response.
(The recent Replicel shareholder update stated stage 1 of this research “has successfully completed (details to be announced shortly)”)
Presumably if Replicel can isolate what particular gene / protein expressions yield better treatment responses for their treatment, this might bring Shiseido back to the table – as otherwise Shiseido would be limited to using only the earlier Replicel technology covered in their original agreement.
So it will be interesting to see both the results from the University of British Columbia joint study and the Shiseido study when they are announced – and how this may all play out.
Excellent analysis!
Great comment!
excellent breakdown
This basically answers the question you posed in the following post. Investors see an argument with one of the largest companies in Japan and run a mile. Any reasonable analysis of the information publicly available shows Replicel are at fault for not holding up their end of the bargain. This is a poorly run company with a decent-ish product. Without Shisheido’s backing i have lost all hope for this product.
Damn. Dropping knowledge over here.
Nice work Xtra. Hopefully the results from the Univ. of British Columbia are released within the year.
They should just release whatever they have, even if it’s just maintenance. Too much bs with hair loss industry. Why does everything have to be so exhausting with this sh*t.
Haha
They have every incentive to meet their 2022 target, otherwise they won’t be first in Japan. Riken comes to mind.
I suspect this year will be make or break for Replicel’s RCH-01 depending upon the results.
I live in Vancouver where the University of British Columbia is – there were no human trials at UBC , so I’m curious where all this talk is coming from ?
They ( Replicel’) did do a trial a few years back on Achilles’ tendons and I actually tried to get in but didn’t qualify . At the time , I spoke with someone who was overseeing it and was at an arms length distance from Replicel . He was involved in making sure their trials were ethical and run propey .
I questioned him and he was somewhat open about Replicels technology, to me he didn’t seeemed convinced it was sound for both hair and the Achilles .
Put it this way – he had already seeem preliminary results and he was not all That excited . Ever since that conversation, I’ve never been convinced Replicells science is the answer,
Im guessing , it does something, but for myself , it probably wont be a game changer .
Hi Stu,
I have no “inside information” – the reference for the UBC study came from a Replicel news release, June 13, 2017. (See link below)
I believe the study intended to use pre-existing data from Replicle’s earlier trials rather than conducting new human trials.
Was interesting to hear the views of your friend / associate who was somewhat involved in the Replicel tendon study!
Cheers.
https://replicel.com/news/replicel-collaborates-with-university-of-british-columbia-to-build-world-class-hair-follicle-cell-data-map
Yep great work Xtra and all other comments -been waiting for this post admin ..:and will be eagerly waiting for more as I think this regent stuff is the cure!
Latest puretech presentation briefly mentions follica stating should have clinical readout very soon for optimization study and begin next study shortly after pending results. At the 25:45 mark of the cowan presentation. Again very breif but nice to hear still in the works
http://puretechhealth.com/reports-presentations
FYI, George is a speaker next month at the 11th world hair congress, maybe some news. Also Samumed is one of the main sponsors.
Hi
These are all show off !!
nothing is ready replicel and shisido do it because they are out of schadule
I think the “If Shiseido decides to do so” comment was just them not wanting to speak for Shiseido.
They have a history of playing it close to the vest and not overhyping, which I actually respect
Hey Admin,
http://www.rapunzelbioscience.com
Looks like Dr Angela Christiano has got the ball rolling on her company.
That site has been live for a year. No new additions in that time.
That Rapunzel site sounds promising. The same idea behind Tsuji is it not? Does Christiano have cultured hair cell science up to where it needs to be to start performing the so called “cure” of unlimited donor area hair transplants?
Hi Admin,
What’s your take on this HairClone (UK) Reddit AMA?
https://www.reddit.com/r/tressless/comments/a65uew/hairclone_developing_cell_replacement_treatments/
Although I highly appreciate their CEO taking the time to answer questions, I find it an absolute joke they have the audacity to tell people to crowdfund them with no proof of concept. Another thing is the involvement of a certain hair transplant doctor tells me this is nothing but a marketing exercise for his UK clinics.
Hey guys has anybody used rogaine? What are your experiences with it? My hair just started thinning should I wait to use it? How is the dropper or foam? Which is better?
I use the Regain foam and have found it has slowed my hairloss over the last 4 years. You have to be consistent with it. It’s not a miracle and i’m still losing ground but definitely at a slower pace.
Never ever wait. Get on treatments immediately. I also use Nizoral shampoo which supposedly helps.
I used it for 9 year, but stopped giving benefits the last few years. My heartbeat increased to the point that I had to stop. At that point i had a massive horrible shedding and thing kept going downhill. My advice is don’t even start that crap.
A couple weeks ago there was a really, really good article about Christiano: https://magazine.columbia.edu/article/radical-solutions-baldness
Some Quotes:
“She quickly learned why there was so little progress on hair disorders. ‘Institutions wrote back to me saying, in essence, that hair loss was trivial, cosmetic, insignificant in comparison to AIDS and cancer.”
It had been fifteen long years since Christiano discovered the first gene for hair loss. […] “I’m happy to say that this year, for the first time, we’ve finally found some genes.” The room got quiet. Then people stood up. They began to weep, and, in a show of unity, they started taking off their head coverings. Christiano, unprepared for any of it, dissolved into tears.
Christiano learned more about the feelings that surround hair loss. “Because the condition is usually progressive, once it starts, people really struggle,” she says. “I’ve heard people say: ‘I couldn’t get out of bed.’ ‘I will not take off my hat.’ ‘I won’t go out in the wind because I’m afraid my prosthetic will come off.’ ‘I won’t be intimate with someone.’ ‘I can’t go on this job interview.’ ‘I’m afraid to show my family.’
“You need to walk a day in the shoes of someone before you cavalierly dismiss AA as ‘just cosmetic.’ It’s easy to trivialize it. But people are desperate, and vulnerable to the core. They’re up all night reading about hair and counting the hairs in the drain and looking in the mirror. You can’t understand it unless you’ve been there.”
[of JAK Inhibitors for AA] The drugs are still in clinical trials and could gain FDA approval as soon as 2021.
There has never been a truly successful treatment for the most common form of hair loss, androgenetic alopecia, better known as male pattern baldness.
FROM ONE, MANY
“Experiments are underway, and the evidence has been tantalizing. In theory, that little scallop of scalp floating in the dish at the Christiano Lab could be the source of a full head of hair. Working toward that future, Christiano and her team harvest the cultured follicles and implant them in artificial skin built in the lab. This skin nourishes the follicle, prodding from it a colorless, keratin-protein filament. The team then removes the productive follicle and grafts it onto the skin of a mouse. The result? Hair growing out of mice — human hair.
Christiano expects to move these trials from mice to people in two to three years [2021-22], bringing nearer the day when people can grow, in a lab, their own viable hair follicles — a limitless supply of one’s own hair.
“From one, many,” Christiano says. “That’s a beautiful technology.”
[!!!]
Hey Bekoo, you’ve posted the link twice which makes it a broken link.
https:/edgy.app/researchers-discover-new-hair-loss-prevention-technique
So trials STARTING in 2021-22, running for a solid 5 years if not 7 in total…talking 2025..pathetic
Don’t worry guys, the cure is only 5 years away.
2024: Don’t worry guys, the cure is only 5 years away.
2029: Don’t worry guys, the cure is only 5 years away.
I seem to recall a posting from Replicel that Shiseido was waiting for Replicel’s dermal injector to hit the market first before proceeding with bringing the hair loss “cure” to marketplace. If that is still true, Replicel announced today that the injector will be released next year in China, Hong Kong and Europe.
Results from Shiseido’s study of RCH-01 in Japan will be released within two months (as of posting) as per personal email from Dr. Tsuboi, who lead the research. No word on market launch or effectiveness however Shiseido recently redesigned the R&D section of their website and it includes the Cell Processing and Expansion Centre (SPEC) in Kobe front and centre on a map:
https://www.shiseidogroup.com/rd/collaboration/
In my opinion, if it was a dud, this would have been quietly removed. The fact it is there makes me optimistic.
If RHC-01 was really promising, Replicel’s stock wouldn’t be at .25. And they’d have no trouble getting more funding. :(
Guys better wake up and I say this with a heavy heart. Unless you get hair replacements like Scott or hair transplants, there will not be a cure for baldness. In 2020 do not expect a cure and truly that is sad.
I am more hopeful of the recent news.
The below link has been shared on a stock forum. I have included what the guy posting it has put as well. Can a cleverer person than me explain this?
https://www.j-platpat.inpit.go.jp/c1800/PU/JP-2019-058164/60AB88FAF5C4DAC7C46914179D50FFAF571630E1250B4FCEE674882A08D3B041/11/en
Admin: Sorry, I had to delete much of this comment as I do not want people to buy or sell stocks based on some pre-existing patent. No guarantees that they will come out with this product, even if they saw success in mice and humans. A few other companies have shown such optimism in the past, only to fold or keep delaying for years and years. I guess that even by writing these comments I may be influencing people one way or the other. So my final thought is that just always be wary and make your own rational decisions.
No problems just thought it might be of interest get your point though wasn’t meant as a sales pitch.
I did not take it as such, and thanks again:-)
I have been following the hair loss world for what must be close to 20 years now. Disappointments galore despite patents promising heaven.
Agreed, you do great work though I’d have never heard of half the possible solutions on the horizon if it wasn’t for this site, fingers crossed one pays off soon
Since when did it become cool to be Eeyore?
This is good news. Hair loss is one of the most complex medical conditions, certainly among the common ones, that humans deal with. We have gone from terrible looking transplants in the 1950s to a potential cure a few years away at most and maybe even sooner.
If you aren’t satisfied with the pace of progress, why are you on this forum and not in a lab developing a cure yourself?
Now that we are almost in 2020, I’m wondering which potential treatment / cure is most promising. What do you think, guys? Admin?
I’m going to come out of left field and say that, for results now without waiting for newfangled technology, Botox injections into the scalp. I read the materials at perfecthairhealth.com and the basic idea is that mechanical stress to the scalp causes fibrosis around hair roots which causes miniaturization. There are plenty of studies involving Botox that validate its effectiveness and a number of site members have used a scalp massage technique that loosens the muscles.
Mind you, if you want to actually get your hair back, you’ll need to supplement mechanical relaxation with wounding or PRP. But I am going to be trying the Botox approach. I will report back any results as they happen.
I’ve also taken a lot of interest in botox for hair loss myself. I am scheduled to have the treatment carried out on friday. More info here: https://www.hairlosscure2020.com/botox-for-hair-growth/#comment-162597
My thoughts are that if they really had something here there would be urgency to get it out. Delays have always amounted to nothing in the past.
If an affordable cure comes out in the first half of the 2020s then I guess we would be hitting jackpot.
The chances of it being released in 2020 are pretty much null. But nonetheless the admin should keep up the great job that he has done thus far.
Shiseido is the cosmetics equivalent of IBM. The wheels turn slow, but they do turn. My hope is they have cracked it and the delay is for legal/red-tape reasons.
The recent “photo proof” by Aclaris and Follica clearly tells them there is no rush.
Scott it’s nice to try and be optimistic but with all due respect, its wishful thinking. Either way, happy thanksgiving.
Wanted to give a shout out to all my brothers and sisters today that are meeting up with families or friends or whoever and will feel obligated to try extra hard to do something with any hair they have left to try and not stick out like a sore thumb. I wont be eating with anyone but there are many on here that will feel your pain. However enjoy the food and try and relax!:)
Quick Bit on Minoxidil:
Guinter Kahn, a balding man himself, discovered the effectiveness of minoxidil as a hair treatment in 1971, but couldn’t take it because he was allergic to it.
Minoxidil, at the time, was owned by a company named Upjohn. When they were informed by Kahn about the hair growing potential, they reported Kahn to the FDA for conducting unauthorized experiments, then secretly filed a patent for Minoxidil as a hairloss treatment and sat on it. Rogaine was finally FDA approved in 1988 for hairloss.
In ’89 it was deemed a spectacular failure. Snake oil hair loss remedies were outselling Rogaine 10-to-1. [Upjohn] believe the blame is on consumer’s expectations being too high: they warned from the start that the tonic wouldn’t work at all for most men.
Tragic irony, corruption, and deception. At my most cynical, it sure seems like the hairloss industry hasn’t moved beyond the snake oil salesmen, only the language has changed from the ‘super miraculous see-it-for-yourself’ theatrics to stoic presentations in webcast graphs and biotech buzzwords.
Hi Tocatta, I’ve been saying this since day one on this site and I always get back lash for saying a cure will never be released. Look they knew minox worked since 1971 and didn’t release till 1988. Wtf. They have probably have much better working treatments that are just sitting in some lab. We will only get treatments like follica, fin, cb, prp etc that just gives you slight growth. If you want more hair get fue. They will never release a product that will outperform an ht. Ht docs will be the ones to perform cloning if ever released. So all these new drugs in the pipeline are maintenance and slight regrowth. Imagine if follica could grow your hair back to me status. Who would go get an ht done and get all cut up, hide for 2 months during healing and spend 20k. Nobody. The industry won’t allow a treatment to outperform ht. The next day ht clinics would shut down. It’s all about money! I know I have turned sides on follica, but I wouldn’t be surprised if they could turn a nw6 to a nw1. Maybe they have done it but will only release a treatment that works 3x better than minox. Then in 10 years they release the new compounds that will work 3x better than follica 1.0.
You don’t get back lash for believing a cure will never be released. It’s a valid viewpoint. Like the scientists of the 1800s that tried to turn copper into gold, it might not even be possible, in any amount of time. They have tried since ancient Egyptian times 5,000 years ago. If all it took was a bit of raw seeds, onions or micro-needling you would think they would have figured it out by now.
The reason you get back lash is you believe in all that illuminati/conspiracy rubbish that in secret there is a cure to hair loss and probably cancer as well but the evil rich suited men won’t let you have it. They are also apparently deliberately injecting autism into children and turning the frogs gay.
May I suggest the Alex Jones channel.
Scott don’t be naive my friend lol. If you think they cant cure cancer or hair loss then you are living in the clouds. They can grow vaginas, kidneys and bladders and have actually implanted them into humans. You are telling me they cant grow or 3d print a follicle?? Come on dude just stop. THEY CAN! They won’t release it because we are all desperate and the rest of the millions of other people who will use cheap snake oils and big three will huy this stuff. They make billions just like the weight loss industry. It’s the same desperation. You cure weight loss there goes a billion dollar industry. All we will get are mediocre treatments that will give us short term or weak results to keep us somewhat content. People get excited for 5 new hair strands lol. Industry feeds off this desperation and profits like crazy. Why release an expensive cure for only few to afford one time and be done when they can release follica, prp, min, cb, sm, to millions for their whole lives and make billions. As you always say follow the money….
Don’t agree with the HairLoss conspiracy and I believe many conspiracy theories due how corrupt the major gobs clearly are. However, everyone is afflicted by baldness even Donald Trump. If he could print hairs he would. This is however why I think there will be a fix cure (3D printing or multiplication) before a magic pill or potion that truly effectively Prevent hair from falling out. There’s so much more money to be made from a patch cure than from propecia etc. Ps the government killed Princess Di. :)
Jesus Christ, is this a hair loss site or a conspiracy forum? Hey, let’s talk about how the oil industry is suppressing a small device that can make your car get 100 mpg while we’re at it. I can only assume if you are claiming this paranoid nonsense that you don’t actually have hair loss and are just trolling the more gullible members of this forum so they despair. Well, bravo.
I was the back lasher and remain to be so much that I’m actually a fan of that conspiracy because its just so hilarious.
I checked this site in hopes of a Thanksgiving miracle. Ah well, there’s always Christmas.
This isn’t a hair loss site anymore because there isn’t anything new coming out worthwhile lol. We have to argue about conspiracy theories to keep ourselves entertained haha. It’s going to be awhile before a real regrowth hits the market…2030+.
As for the oil industry they definitely have a huge hand in lobbying to meet high mpg engines on the market . That’s not a conspiracy theory it’s well known lol. I went to bmw factory in Germany and spoke with a lead engineer there who developed a 85mpg inline 6 engine for their 3 series. They said they could never bring it to market. Said electric will be the future for bmw, porsche, Benz for their fleet.
I’m with MJones in believing–well, ‘hoping’ better treatments are in a lab right now—aren’t we all. I mean, that’s why we’re here! And it doesn’t seem conspiratorial when one realizes that superior treatments have been shutdown by at least some type of suppression in the past. Two examples come to mind:
THE STRANGE PREDICAMENT OF RU58841:
https://www.hairlosscure2020.com/the-strange-predicament-of-ru58841/
DR. RICHARD LEE — Minoxidil 15% plus Azelaic Acid:
https://www.hairlosscure2020.com/category/azelaic-acid/
I don’t know about secret cures and all that, but there was a Cambridge scientist, Regina Hodits that had a cure for the common cold, “but when we discussed this with pharma partners, they were not amused. They thought that the cost of such a treatment would be too high for ever being considered for development.” […] “That event told me that all my science wasn’t actually going to see daylight if I didn’t know anything about economics.” […] “People have become much more disciplined on saying ‘how can I invest into something that, in the end, is going to turn into a product?’”
How much different would our perspective be if instead of hairlosscure this was just hairlossproduct.
Is what it is.
(English Tsuji article from 2019 confirms first human clinical trials will *begin* in 2020. 8. Conclusion and future perspectives: https://royalsocietypublishing.org/doi/10.1098/rsob.190010#d3e1016)
Regarding RU58841, this was 4 years ago and several hair loss sufferers got their hands on it illegally to combat hair loss. Where are they now? Where are the viral YouTube videos of these newly Norwood 1 formerly bald people? Either it didn’t work, or the illuminati had them all killed to keep their hair loss cure safe :-) :-)
As for Regina Hodits, all things have to make economical sense or they don’t happen. There are so many bogus university projects out there. A cure for the cold will mean losing opportunities to call in sick for work; people wouldn’t take it if it was given out for free.
On the other hand, a cure for hair loss made by an individual would be so economically viable it would make that person one of the richest people in the world.
Scott a cure for HL would surely make me feel rich!
https://www.prnewswire.com/news-releases/breakthrough-gene-therapy-clinical-trial-is-the-worlds-first-that-aims-to-reverse-20-years-of-aging-in-humans-300963496.html
Gofundme egghead need 1mill hit me up
Not asking much just 1 mil
The hair loss industry has known for decades that a DHT resistant hair follicle could be implanted anywhere and grow forever (simple as a plant in soil). Therefore, a hair loss cure would be to simply clone follicles in decent numbers. Entire animals have been cloned for decades. China has made genetically modified babies and human monkey hybrids. Body parts and tissues are now being 3D printed. If the industry was truly motivated to clone a little hair follicle as a viable product, they would’ve done it by now. The problem is that eliminating bald people with a one-time treatment would be terrible for the wig, finasteride, propecia, and current HT industries, where they love getting years of recurring payments. In 2007, I saw predictions on CNN and Popular Science that baldness would be cured by 2009 and 2012, respectively. The technology was there, but it vanished. The lack of progress since then has been astounding. Clone a
some back-of-the-head follicles… That’s the only true cure, and yet it’s so very very unattainable for some strange reason.
Scott, cures are not discovered by individuals. An enormous network of research, clinical and scientific teams and advisors, engineers, marketers, directors, and executives; company partnerships, corporate alliances, biomed institutes, private investors, blah, blah, blah, go into developing a treatment. You don’t need a coterie of evil men to be thwarted. Just indifference.
“Something as simple as lack of sufficient funding despite successful stage 2 clinical trials can cause a company to stop pursuing highly promising products.”
—Admin said that five years ago! (Nov 25, 2014) And guess what we were all hyped about: Histogen and Replicel! https://www.hairlosscure2020.com/lessons-from-aderans-and-intercytexs-hair-multiplication-failures/
(Read the comments)
Where are we gonna be in 5 years? I thought I knew, but honestly, I don’t.
Maybe it is the government’s conspiring against this as the facial recognition would all need revamped due to so many people looking completely different.
Sorry admin, haven’t posted in a while and don’t recall my screen name.
Thanks for the wonderful blog!
Thank you Slick and Tocatta for supporting me. You both said it perfectly. They can grow clone print etc yet the follicle just can’t be done. They just don’t want to release it yet.
Despite your pessimism, you were always optimistic about Follica and Cotsarelis! I thought Cotsarelis was part of both the Illuminati and Masonic Lodge and heavily involved in the conspiracy too…
Hi Admin, yes you are right. But after those results I am extremely disappointed. I’m hoping these are just teaser pics and they release better ones later. As of now, I’m very disappointed in follica.
If you guys really believed that a cure was being suppressed, you would not be on the internet complaining. You’d be teaming up and kicking down doors. Give me a break.
So guys what % of hair loss do you think propecia can grow back. When I say, propecia, i mean finasteride. Might take a topical from a Dr. I did take finasteride for 1 month. I didn’t get any sexual sides….but the first day I had bad breath. Really bad, it smelled like when if you get a filling and they drill on a tooth. I brushed my teeth several times that day….nothing could get rid of the smell lol. Then in Lab before my clinical rotation, I noticed my brain was as sharp at all that month. I have a 3.8 G.P.A taking Pharmacology, Patho physiology, Anatomy and physiology, physics, nuclear chem etc…all the hard “core” sciences. So when my brain was not on point, that’s when I got off it. I took it orally, in the form of proscar, and just bashed it into 4 to 5 pieces.
So I never gave it a good shot. I am a Nw 2.5ish. So idk how good finasteride would work. I started 4 years ago with hairloss. The one thing that did happen, my hair stylist thought I died my hair jet/dark black. My hair got slightly darker on proscar. So what % did you think topical fin could grow back ( reverse miniature ). How effective has it been for you guys within the first couple years. Again I am around a 2.5 NW with it happening 4 years ago.
0%
edit: I noticed my brain was NOT as sharp. Typo, at the beginning.
If you are a Norwood 2.5 I would say forget about it and go out and live your life. Find the love of your life, travel, study, party, get rich, whatever. Then come back here in 5 years. We will either have a cure for you, or there will be less players to read up on.
I am a Norwood 5. But courtesy of surgical scars on my head if I take my hair systems off and have an army style hair cut, I don’t just look old, I look like the villain from the blade 2 movie.
Norwood 2.5 – I don’t even consider that loss. You can’t look like a 15 year old forever.
Are your scars from failed FUTs?
No. Scars nothing to do with hair loss. The FUT scar was massive when I first had the op and I was worried, but its not visible at all. I am grateful for that.
Thanks for the reply guys. I should mention my 2.5ish NW is diffuse. It makes me feel like a 3. The back of the head on crown is thinned and got recessed on both sides. Nothing close to say Jude law..but…if I comb my hair like Brad Pitt ..on The movie Fiery, 2014 world war 2 movie, it’s hard to tell I have hair loss. When it’s wet though…ouch. Pitt brushes his hair to the side on that movie and back. However, my hair loss was do to a medication, and it never reversed. One doctor seen my blood work and said the previous physician had me on too much and it was clear in my blood work why I lost hair. I won’t lie, my confidence took a big hit. I stopped lifting weights…became a shell of myself. Last time I went out, got hit on etc…but idk. I had a very very low hair line.
Forgot to add about diffuse.
Yea if I were you I would say forget the pills and lotions and work on kicking butt in school. You will regret it later if your grades slip due to being on a treatment. You seem to have plenty of tools to snag an attractive mate and do well in life.
Hi Derek don’t listen to Scott. Horrible advice. Hop on 1mg of brand name Propecia. If it works for you it will hold your hair as is at nw2.5 for at least 10 years. I started losing my hair at 20. I hopped at the first signs of shedding..nw1.5 diffuse. My hair loss stopped within a few weeks of using it. I got thickening and regrowth around 18 months later. The thinking areas filled in again. Not 100% but it wasn’t see through in the sun anymore. I used Nizoral 1% shampoo and country life Maxi Hair vitamin. I work out 4x a week, eat healthy. That mix stopped my hair loss for 12 years. All the men in my family are nw6 to nw7. So very aggressive. I did get sides for the first 6 months. Libido and some brain fog. That all went away after 6 months. I would not use Rogaine if you aren’t on Propecia. Without Propecia you will most likely go bald. So if you don’t care about hair loss and are content with losing all your hair then take Scott advice. If balding bothers you, makes you sad or stresses you out then hop on Propecia. 80% chance it will stop or extremely slow down your balding. The sides you mentioned about bad breath is the first I heard of. Topical won’t work as well as internal. Take brand name Propecia 1mg for a year and see how it goes. You’ll get some regrowth if you just started balding within the past few years.
Also you can travel, work, find the love of your life, hit the gym, study while being on Propecia. I did :) also don’t ever bank on a cure being available in 5 years. I went to a ht clinic back in 2002 and they told me to hop on Propecia to hold on to your hair till 2010. A cure like hair cloning will be available then. Here we are 10 years later and we are still using the same big 3 drugs lol.
Yes “only use brand name propecia” all others are inferior. They definitely won’t mess up your endocrine system. You can trust me and the “official brand named propecia”.
“Official brand name propecia, is the only answer to most of life’s problems”.
Please note I am not affiliated in any way the worlds best treatment for all life’s ills “official brand named Propecia”
“I am definitely not a shill for the company brand named Propecia”
Was this advice or sarcasm? Not being sarcastic, just asking.
I’ve always used cheap finasteride prepared for me by my local pharmacy “15 years” 30 euros for 100 pills and I’m doing just fine, never had any side effects.
I would of thought hair cloning would come before that magic injection or pill that actually regrows your hair back – Still waiting on those. Other commenters have said we can clone all kinds of stuff but hair follicles are tricky. Once they figure it out and have these hair farms – Hair transplant surgeons are still in business (getting tons of work), donor region is left untouched and no scars, patients are able to get insane density. I’ve been thinking about getting a HT but I want that to be my last resort. I’m able to style my hair a certain way that gets me by for the moment (thankful for that). I would honestly love to just wake up and go to work without having to style or worry about my hair. I’m currently taking brand name Propecia, 1mg. I was on generic but made the switch recently. If your thinking of taking fin, see for yourself if its for you. If you read and watch every video on side effects, you’ve already planted that seed in your brain. Trying to remain positive that something decent or amazing will come out but by no means counting on it. To all my brothers and sisters going through this hair loss journey I solute you.
We solute you too my brother
Hi Admin, thank you for your update. I have the full PDF of the trial Shiseido (25 pages). How can I share with all of you ?
You can email it to me if you want. See the Contact page of this site for my e-mail.
Hey :) I’ve just send you an email with the PDF. I hope there are some good informations. Cheers
Thank you!! I will try to read it tonight or tomorrow morning.
Thank you to you Admin for this great website! cheers
If they do find that you start to lose the new hairs at 12 months, I wonder if Finasteride could help combat that…?
Admin, when you get a chance can you please take a look at this I think this is good news for Dr tsuji and others. https://yaledailynews.com/blog/2019/09/17/hair-follicle-regeneration-may-prevent-cancer-growth/. Because I remember people saying hair cloning May cause cancer mutations. I found it very interesting when it said in the article “the researchers hope to elucidate why a hair follicle is able to suppress tumors“
Shisiedo are listed as the source of funding for this study.
Are there any numbers in the study for increase in density or Target area hair count etc?
I dont really want to have to pay $35 to read it.
So, the cure fore hairloss indeed came in 2020
https://gramho.com/media/2233081671372250791
They claim these are exosomes therapy results, great outcome in just 5 months if true.
@faust I saw those results on Instagram and the clinic that injected that guy with exosomes also did micro needling on his scalp there’s a video of one of the nurses doing the micro needle pen device on his scalp.
And now I am going to drink a big glass of I told you so; glug, glug, glug! This should act as a warning to the other players still in the game.
The Japanese have a historic industrial habit of copying something, making it more efficient, then selling it everywhere. It would appear they have done the same with Replicel’s tech.
I may have misinterpreted, but even if this cheating is true, I don’t care. I just want my hair back and will give my money to whoever makes that happen, even Elon Musk or that bald villain who runs Amazon.
This indicates they won’t commercialize based on this study:
In conclusion, this clinical study of autologous cell therapy using DSC cells to treat male and
female PHL has shown positive, although temporary, responses at the lowest cell concentration injected, and further studies are warranted to determine the best concentration of cells and treatment regimen.
In order to determine if this cell-based treatment provides a significant clinical change noticeable to
patients and practicing physicians, additional clinical studies injecting DSC cells in larger hair shedding
areas should be performed to demonstrate a visible effect by global photo-assessment.
So the last line of the new update maybe shouldn’t be in the post.
So in other words this treatment of sisheido needs more trials due to lack of results. Gains diminish at 9 months and at the very end it stays for this to be commercially available to doctors it needs to show visible results. So this probably just grows a hair or two. Lack of pics, weak results….and you still need propecia for this to work….bs. The whole purpose of this crap was to inject your scalp, get robust regrowth and maintenance and maintain for 5 years and then repeat. NEXT!
How far we’ve fallen from that first promo video that projected a full Norwood 5-7 recovery. : (
Is it safe to say Replicel/Shiseido RCH-01 was a complete failure and a waste? I guess we should hope that HairClone and Tsuji will be successful!
@C correct me if I’m wrong but I believe hairclone’s thing is like the same thing as RCH-01
Nope,
Hairclone are using DP cells where Replicel/Shisiedo are using DSC cells.
Hairclones approach is more similar to what Aderans were doing just with improved tech and a different objective (follicle rejuvenation instead of follicle regeneration).
Hopefully it’ll work out better.
This is disappointing.
For me, something like RCH-01 was (is?) an exciting prospect. I really hope the next effective treatment is something like RCH-01. I find it very difficult to get excited about things like topicals or microneedling.
For me, unless the results are remarkable and genuinely noticeable, then the daily hassle (putting solution in your hair twice a day, needling your scalp once a week) isn’t worth it.
L
I really don’t understand what this all means
Can’t they just release it and work on perfecting overtime. I’ll take anything.
No pressure, Dr Tsuji….
@haironmykeyboard Let’s Hope Dr tsuji doesn’t get coronavirus.
Wait a minute so they said that the the positive effect was temporary till 9 months. This is a bit vague, does it mean that your hair will start falling after or that you don t gain as much regrowth further as before? I thought that RCH-01 was made up of DHT resistant dermal cells so it should be a permanent solution tho.
Here’s how the data presents (Hair count per cm2):
—At 3 months, you’ve lost 1 hair per cm2.
—At 6 months, you’ve gained 3 hairs per cm2.
—At 9 months, you’ve gained 5 hairs per cm2.
—At 12 months, you’ve gained 1 hair per cm2.
So after a year you’re almost back to baseline.
Hair diameter is also back to baseline at 12 months.
High Norwoods 5 & 6 lose after the first 3 months, recover to baseline after 6 months, and make a slight 1 hair gain by month 12. They basically get nowhere.
The researchers also never directly injected into the bald / thin spots (temples and crown) but on the edges; four injections like this :o: —So I don’t think they had much faith in reviving slick or even thin areas. It looks pretty dense already in the phototrichogram images.
Hi Toccata, -1 +3 +5 +1 = +8. So how is that almost back to baseline?
I’m always counting from baseline. If you had 20 hairs per cm2 pre-injection, you’d progress through the months like 20-19-23-25-21. You started with 20 and ended with 21. Call it maintenance for rich people. :P
Thanks, makes sense. Hopefully they can test other dosage iterations and then once every 9 month injections could mean more than just maintenance.
@admin are you able to contact shiseido for their take on this and their current hair pipeline?
It still doesn’t really make sense to me either
Personally, I think treatments such as Shiseido’s are still about 10 years away from being commercially viable. In the short term, Tsuji is probably the best bet for a “real” cure, but it likely won’t be affordable for the vast majority of people. Even for those who can afford it, there may be some travel restrictions to and from Japan so that Tsuji and his team will be able to monitor the results.
An FUE transplant still seems to me to be one of the best options in the near future, maybe until 2025-2030. Especially so if you have enough hair left for the transplant to give the illusion of a full head of hair.
Hopefully you won’t read this as overly pessimistic! I strongly believe that there will really be a commercially available cure by the end of this upcoming decade (2030). But in the short term I wouldn’t hold off on a transplant for hopes of a cure coming around the corner.
I don’t think so. While I agree that it won’t be affordable initially and you won‘t get it outside of Japan initially – it won‘t take another 10 years. 2 – 3 years after the japanese release should be realistic.
They will definitely target Europe and the USA and will find ways to offer it there. OrganTech‘s mission statement on their website also says sth like „offering it to the world“. The major players Itochi, Mitsui and Kyocera want to maximize profit, that‘s certain – and that’s good for us and only possible if they offer it worldwide.
I certainly hope so! Assuming a 2021 Japanese release at Tsuji’s price of ~ $200,000 – $300,000, it’s totally possible that we could see the technology offered in countries outside of Japan by 2023-2024. Literally everyone everywhere will want a piece.
I’m still thinking that the price will be somewhat unattainable until 2030, even if it drops to $100,000. I guess it would also depend on where you are on the Norwood scale and which surgeon you choose (for your “unlimited” grafts). However, since the new replication technology would likely be sold to transplant clinics at a fairly high price, I would assume prices for cloned grafts would be significantly higher than regular FUE. For higher density or a larger number of grafts even the cloned hair would require multiple sessions with a surgeon.
That’s why I’m personally hopeful for a real “cure” in the next few years as well as Tsuji’s cloned grafts. It would likely be cheaper than surgical solutions and possibly drive the price of cloned hair down.
FUE is probably king for the next 5-10 years, but if we see a better solution sooner I would be absolutely ecstatic!
It would be really interesting to get more insights of the efficiency of this study. And actually one „conspiracy thought“ I always had regarding the reason why shiseido has delays is because they search for a way to modify this treatment so people would have to get injections again and again instead of offering a one use treatment and final cure. Wouldn’t even care as long as it’s working.
Admin can you please check this article?
I expect news about Dr. Tsujis hair regeneration!!
https://premium.toyokeizai.net/articles/-/22606
Its from January, but there is a paywall to read the whole article. They say trials beginning soon.
https://gramho.com/media/2233114120773142006
Hey, I’ve found new some new exosome results, older guy had great regrowth in 50 days (they didn’t specify how many treatments)
https://gramho.com/media/2233081671372250791
This is another guy, impresive regrowth after 150 days (6 treatments)
I think this is shaping up to be the best non invasive option we have ATM in hairloss world. We didn’t see that much coverage, in some interviews doctors and techincians were impressed and suggested that exosomes significantly outperform every other injectable.
Purity and safety are main concerns (with price), after that dosing, method of application etc..
What are your thoughts guys?
Had exosomes done in June by one of the reputable names you’ve seen. Did absolutely nothing. Like, less than prp. And I’m the perfect case for it. Minimal hairloss. Just needed slight density at the hairline. A costly experiment. But, that’s just my experience.
Exosomes are el dorado for scammers, dark market, where you often don’t know purity, source of exosmes, etc.
Having said that, price is not product of demand but of source, extraction process and underdeveloped economy of scale.
Back to efficacy, there are no detail studies exploring dosage, frequency, method of application, and possible different source of exosomes. For instance XoFlo derives exosomes from mesenchymal stem cells from umbilical cord, and they are cheaper ways to derive these cells (DP cells for instance), but we don’t know how potent they are, or their efficacy as hair regenerative treatment.
I think results, if true, are quite impressive to be honest. If price goes down by a factor of 2 or 3, with little bit of research I can find the right clinic, then I’m definetly doing this.
For women, like myself, this is very discouraging news. We need treatment like yesterday. We need our hair to function. I feel like there is no hair loss cure and never will be. I hope that Shiseido has another product coming out on the side because this is very embarrassing for their business and reputation. There stock will likely go down now. Shame on them and all of the other companies for giving us false hope and intentionally misrepresenting that they hold the cure just to get exposure. Hairline and the other two companies remaining from Japan need to shit or get off the pot and not play games with vulnerable people. I went to one of the most well known prp doctors several years ago and got his extra cellular amniotic prp injections. I got the worst allergic reaction that has lasted years. There is nothing out there.
I feel your pain. It’s bloody terrible looking in the mirror everyday seeing your hair fall and Not being able to do anything about it. Everywhere I go I see people with lovely heads of hair, it’s so unfair.
I keep coming back here hoping there will be some new development. But it’s been going on for years now and it has given me nothing short of depression with the constant let down.
I am ready to throw in the towel.
To all of the people here in depression, I urge you to accept the fact that they grow old and bald. Many of the companies I’ve seen presented here seem like cosmetic Ponzi schemes designed to pump up their company stocks rather than legitimate hair loss cures.
Nothing has changed … they still try to sell us snake oil, with a more scientific approach … but it is still snake oil … very sad. If you are one of those who still believe in a miracle cure and you are disturbed by what I say… well go to hell … I have been suffering from baldness for more than 25 years, I am sick and tired of this s.
@admin this bit is optimistic
“In any case, we should prepare for an eventuality where we need once-a-year DSC injections. Hopefully, Shiseido succeeds in rapidly commercializing this autologous hair growth treatment in regulation friendly Japan.”
Question for admin and all you hairloss experts:
I’m 39. Diffuse thinning on top. Over the last few years, I’ve also noticed that I have less hair on my legs than I used to. Less chest hair. My stubble isn’t as dense as it once was.
Now, is this a case of “You are getting old, your hair is just going away gradually” or could this be something that needs addressed? I still have leg hair and chest hair, and I can still grow a beard, but all these areas are nearly as hairy as they used to be.
A few years ago, I got my thyroid checked by my GP, but I think it was a basic check. Anything else worth checking?
If you are genuinely concerned about your health, I would go see a doctor. Not people on an anonymous chat room, not a Youtuber, an actual doctor. Then for the purposes of enlightenment, tell us all what they said.
If you are taking Finasteride or Dutasteride, you can lose body hair. If you have diabetes, you can lose leg hair.
It is rare for people to start losing body hair in their late 30s. Usually, you keep gaining body hair at least into your 50s as far as I can tell.
Here’s a link to the full-text manuscript of the Shiseido study:
https://sci-hub.si/https://doi.org/10.1016/j.jaad.2020.02.033
– Phil Collins, PharmD
I read the report and I don’t think it’s a total flop. It states that there was a significant increase. I just think that the whole experiment was a waste of time to drag out for so long and have everyone thinking they would come out with a treatment in 2020. First, I don’t think they had enough subjects. Secondly, I don’t think that they targeted the correct areas for evaluation. Also, they could have worked around some obstacles or done two studies at the same time. I think it was a boring study and they could have done better and worked to improve the treatment while it was ongoing in in order to obtain sufficient results for market release. None of us care about more investigations or have the time to wait for another study and then for them to release the results. If there is improvement, great get it out and improve it while it is on the market. People are willing to get PRP several times a year. Why is that so hard? It’s just autologous sheath cups.
So is Shiseido delaying again?
The outcome is heartening. The effect of the treatment builds up and peaks at 9 months after injection; at that peak stage, the single dose administered 9 months prior yields an additional 5 hairs in the 1cm2 area directly around the injection site. So just one single jab gives you a peak of an extra 5 hairs in the cm2 surrounding the spot you got the jab in, and it takes 9 months from the time of the injection to build to that peak. Multiply this across both space and time – by way of multiple injection sites in thinning areas (space) on ongoing occasions (time) – and you’re certainly getting somewhere. Consider too the fact that further calibration and improvement of the protocol is inevitable. Also be mindful of the fact that this treatment will no doubt benefit from combination with the tools we already have in our arsenal, I am thinking minoxidil in particular. Look, if they told me I had to do some initial “loading” of many injection sites per appointment across a period of initial appointments spanning a few months, after which I could just top up every couple of months to maintain, I’d bite your hand off, so long as it wasn’t outrageously expensive. Interested to hear the thoughts of the community on this, in particular a sense-check on my understanding of how the overall regimen might function. I mean, here is a treatment from your own cells which rejuvenates hair follicles, surely this is a positive thing x
Extremely well put. Most people were not expecting Replicel/Shiseido to be as good as Tsuji/Organ/Riken. So all the negativity at this news seems a bit much.
If there is a guarantee that once a year injections even just maintain all your hair, I would think of it as a miracle treatment. A +5 new hairs in every small area injected would be major icing on the cake.
The only reason I am not overly optimistic is that we do not know if this will work every year as yet.
I don’t know guys, it feels like we are trying hard to pretend this was a success. It was a major letdown to me personally, the famous histogen +73% increase to baseline at 12months mark was something i’d justify the hype for, but to me this is just a disappointment, for now at least. This is not game changing as some of us were probably expecting, but maybe again it’s me setting myself up for delusion with too high expectations.
We seriously need a regulation.
They cannot continue to sell snake oils only for getting funds or other type of yearns.
How can we punish this?
I think it’s partly our fault. I talk from personal experience, and when I hear about “stem cell” I get ultra hyped, thinking this is gonna be the ultimate regenerative stuff that brings me back to fulll density and stops me from obsessing over hair. Accepting that the problem is a lot more complicated that we’d like it to be ( healing baldness ) and also that we are not that advanced scientifically as we’d like to think of.
It’s a problem that has always existed, you can manage it, and you are lucky enough to be the first generation of men who might see an actual cure, thats enough for me, no need to be ultra pessimistic, you have reasons to smile
Is it true that Shiseido has its own treatment they are working on independently from replicel?
It is my understanding that this news is based on Shiseido’s own version and not Replicel’s product. Shiseido’s own version has yielded worse results than Replicel’s phase 1.
@admin correct me if I am wrong.
No it is RCH-01.
Check out Replicels recent tweets. They confirm this.
What is Shiseido’s version like? Is there any info on that?
Where has this idea of Shisiedo having their own version come from? Is this a fact?
I read in the study that on average, per square centimeter, patients gain (if I remember correctly) 9-13 new hairs. I guess it’s all relative. To me that doesn’t sound like much.
They need to get these next trials going…
Damn words fail to express my disappointment and sadness regarding Replicel RCH 01 results. I had imagined replicel would have been a density boost or at least a mainteneance treatment for those slowly thinning like me, buying time for when things would have gotten worse till hair cloning is a thing. Seems like we still have a long way to go before any major treatment. Thanks admin for your work
Shu, It is “at least a maintenance treatment”. So far, they are averaging a +8 % hair growth result, so more than maintenance. But might require once a year injections. Hopefully, they can test other iterations (including lower doses) to see if they get better results.
@admin are you able to ask them what they are doing now? If they have something they think works they need to do more trials quickly. Plus they are in Japan which is not badly hit by covid19 so far.
Hi D1, the Japanese researchers almost never reply. They used to do so sometimes in the past, but I think got tired of doing so to so many people. Especially the Tsuji/Organ Technologies team.
I can see your point admin, but realistically it will only be available in Japan for a while and it’s still unknown when, if ever. Yearly trip to Japan + treatment its gonna be very expensive, might aswell get a trasplant at that point. Just my thought, hopefully i’m proved wrong
Admin, how long do you think the coronavirus will delay further trials for Shiseido, as well as Tsuji, and others?
I hope by not more than 6 months at most. If Tsuji has no major news this year, I will be very surprised and disappointed.
This treatment sucks plain and simple. We need to stop hyping it up and making it seem it’s great. Injecting stem cells in your head to only get 8 hairs is freaking joke. Plus who knows if these cells wont become a tumor or worst. Maybe in 10 to 20 years they will have a hair farm like christiano. But I heard that song and dance back in 2001. It’s all giant bs money sucking game to sell bs treatments with no better results than the big 3. All these new treatments aren’t showing anything incredible. All touting at most 15% regrowth. Follica claiming 40% regrowth but based on their pic they released it didnt really seem like 40% unless they are down playing it for a surprise shocking result. I’ve been through all the empty promises, delays, shelving and repeat of all these companies. It’s a giant cycle that goes nowhere. If we get follica, sm in the next two years I’ll be shocked. I’m just going to get a massive fue with dr couto and call it a day.
— It is your own cells, so unlikely to cause tumors.
— 8 percent increase per small area of injection, not per the whole scalp. More like 1000 plus hairs over the whole scalp if they can optimize the treatment.
Anything autologous that works to even maintain is good news. Albeit not as good as what we want and deserve in 2020 :-(
8 percent growth after 1 year!? Really? This is not worth an post. Guys, its getting worse! I am so sick of all these liars and Snake Oils! We are living in 2020 and nothing!
OK, the plan for the next 12 months is Polichem, Medipost, Exosomes and Madison Electro Cap.
If these Hair loss Industry Loosers keep that way the Anti Aging Industry will overtake and we use Senolytics, Metabolism Drugs and NAD Boosters before they realize Hair Loss Industry sucks.
This technology should have produced at least 75 to 100 hairs cm2 no excuses. Rogaine grows 15 to 18cm2 if you are a responder and rogaine came out in the 80s. This is just prp. Exosome is another bs treatment. We need legit coverage of scalp and with maintenance. 2020. Who will fly to Japan to get 8 extra hair per cm2 and have to do this every year. Let’s pray follica shows robust regrowth pics this year that justifies their 30 to 100cm2 regrowth.
I would need 25,000 new hairs on my head. An extra 1000 won’t be worth a trip to Japan.
I would fly to the moon to have my hair back. I would fly to Japan for a guaranteed 8% once a year. I would do anything to feel like a beautiful woman and not like some octopus attacked my head. Lol
Give it a few years and this could be great! All new science needs time to flourish. Be patient everyone, I know it’s difficult.
By 2025 I am hopeful that some of these companies will publish very successful results, and by 2030 I have confidence that a real “cure” will become widely available.
But what if it isn’t? I’ve said it before and I’ll say it again here; I really think that the way to go for now – especially if you are in the lower Norwoods – is a hair transplant. It’s proven and it will give you a result that will last years. Be hopeful and remain positive that a cure will come out. That being said, just in case it doesn’t, I’d go for a transplant now and hopefully not worry about your hair until a cure is released.
a bit off-topic but might be helpful for someone (I wish I did it earlier or someone convinced or influenced me). I am following this blog for 5 years (did not comment as was not much value to add) and after years of topic addiction and experiments with hair system I did FUE procedure some 3.5 months ago and would strongly recommend it for others wishing to sort out / “cure” their hairloss problem today and move on (with no or at least way less obsession about hair).
I hope majority here appreciates that realistically proper solutions that make a difference on Norwood 3+ (i.e. tsuji or even replicel if found effective, etc) are still many years ahead and very likely to cost more (if not multiple) than large FUE procedure does today. Anything involving individual cell treatments would be massively expensive (there are not really exceptions for this rule today with other drugs/procedures).
I am advanced Norwood 5 (even closer to 6) and had quite a large procedure but even after 3.5 months already happy where its going and fine if full head of hair cure comes in 10+ years not to be obsessed about it. In my case it helped to divert thoughts from hair into more useful work /family /friends /sports etc and that’s what majority here is looking for it seems
.
“By 2025 I am hopeful that some of these companies will publish very successful results, and by 2030 I have confidence that a real ‘cure’ will become widely available.“
Lmao ‘just wait another decade bro no big deal.’
Many of us will be pushing fifty years of age by then. Diminishing returns. Also that is exactly what people were saying about 2013 back in 2009. Don’t get your hopes up
That’s why I followed up by recommending getting a transplant now.
Being a pessimist doesn’t help.
If the recent study’s goal would have only been to proof safety of the treatment I would’ve said ok let’s be patient. But and average of 8% increase per injected area is not even close to relevant from cosmetic point of view. It’s very disappointing considering the hype around RCH-01 for the last few years. Especially if you’re diffuse thinner like me It would have been the best option. All hopes for a real CURE, not a treatment that’s not the usual 10 years away are put on Tsuji now. Unfortunately they don’t have a single short term competitor anymore.
Replicel have their one of a kind dermal injector coming to market soon. I strongly believe the next trials will commence once this is available. Also shisiedo could launch this now and refine whilst on the market. Unless I’m wrong Japanese laws will allow this after a safe 2nd trial
You do make an interesting point Paul. PRP and laser caps are totally useless for hair loss, but they are still commercial products. So why not this?
I also don’t think it’s a coincidence that after all this time the injector is getting tested and ce marked at exactly the time shiseido look like they need it. The virus has delayed things for everyone but once we overcome it this could be very interesting. Replicel have an ace up their sleeve and arent as daft as some make out in my opinion
I also don’t think it’s a coincidence that replicel are finishing device and getting it ce marked at the same time these results are released. The virus has delayed things but once we overcome it replicel have a big ace up their sleeve. Shiseido tried to make their own injector and failed. Replicel aren’t as daft as everyone thinks in my opinion
When will there be a new Exosomes update admin?
Replicel has a crap treatment and will market this like a cure to those desperate mpb sufferers who don’t know better. Sisheido and replicel will both make tons of money while their customers will get an advanced form of pro. This should have been a near cure like treatment but it’s just an extra 8 hairs cm2. Stop making it seem like it’s going to get better lol. This is only good for nw1 to nw2 that haven’t lost visible hairloss and can use this like propecia. Big If though since it only lasts 9 months lol.
Eight percent… That s not a big deal guys…
Hi
I am searching for minoxidil cream, i wish you can help
@London. Congrats on your fue. I’m glad to hear it’s going well. Where did you get it done and how many grafts? I’m looking to get one done as well. I wish I got it done in late Jan. I am quarantine now here in the states and it would have been the perfect time to have it done. Nobody would see me and know that got it done lol
@Mjones. Thanks – I wish i did it earlier as well – in my case decided does not make sense to wait for too long and just fix it and move on. I researched a lot and even flew in over last years to see Bisanga, Lorenzo and Erdogan (Asmed) to consult. I must admit was not overly price sensitive for this but ultimately stopped on Turkey (Erdogan) as they could do 5000 in one go (clearly don’t want to do it every year in Europe). I did 5200 grafts, I think was c.12k EUR all-in incl tickets etc (If I were convinced I would get better procedure elsewhere I would def pay more but I did not find better). I had 3 month holiday between jobs but must admit saw multiple friends and ex/new colleagues some 2 weeks after procedure and you will be surprised how no one cares apart from yourself – definitely don’t let it stop you.
just to add – there are cheaper guys in Turkey and pretty sure with same FUE quality (maybe less posh facilities as Asmed was like 5 star hotel). So the cost could be lowered but more research / forum feedbacks needed – a lot of those guys they trained at reputable place like Asmed and start their own clinics. Some of those better not to go but some could be very good value for money depending on everybody’s budget. Takeaway is nowadays there is available good enough solution with limited downside with decent doctors in my opinion (if FUE).
Anyone here on Topical Finasteride or a Min/Fin combo that can be purchased? I was waiting on Polichem (now ALM12845) but I can’t wait another 2 years, if it comes at all.
Thanks guys.
Mjones. I emailed Rahal and sent off my pictures for a consultation. No word. I figured he’d be out of office until post-quarantine. I fell into despair comparing my photos to other cases—I don’t think I can recover my loss, and I certainly can’t afford to go into the $30-40K territory doing so. Absolutely gutted by it all.
Hopefully some monumental news is waiting in the wings Admin. I’m in need more than ever! : )
Toccata I know how you feel. Try n keep your head up.
Guys, what does 8% mean per cm2 actually mean? As someone who is not a specialist in this field, I share the same impression of my peers as it is really disappointing. Even so, the scientist in the article seems really confident about it. Should we take his confidence at face value? Is he confident because it worked, even though on a small scale and now his team is planning to reach 80%?
I tried getting hair system, couldn’t stand it. My hair is fine and diffused all over. My donor hair is also fine.
What options will I have in the future, will I still be able to get hair cloning? Been on fin/laser helmet for 12 years now. Kept my hair, but still thin all over. Need help.
Wait two questions. I’ve been balding since I was 15. 21 now. Been on finasteride since day 1, 6 years now. I still shed everyday. But the past 5 Months, hair loss hit me like a brick. My hair got so fine. I still have a full head of hair, it’s just different now. Wait you said your donor hair is thin, How do you know its thin. Also You’ve been on finasteride for 6 years. Why did you hate your hair system. I can’t wait for hair cloning to come out so I can get off this stupid finasteride.
Admin, this seems significant doesn’t it? Would he do this if the results weren’t promising?
https://www.smarteranalyst.com/new-blurbs/the-non-executive-of-replicel-life-sciences-repcf-is-buying-shares/
I could care less about replicel’s product the results are a joke. They are dead to me.
WOOFY97 2nd clinical trial is great progress. What are the results you speak of that are discouraging?
Slick I was not impressed with the last clinical trial results.
Unless they can produce a product that will deliver 100+ terminal hairs per cm2, Shiseido is spinning their wheels.
How naive is it to be optimistic about Replicel at this point?
In other news, bear sh*ts in the woods…
So in the middle of a global pandemic when money could be better spent elsewhere shiseido have started a further trial. Would they do this if it was dead in the water? Take replicel out of the equation shiseido are no fools so pls tell me why they would bother if there is nothing positive ?
It costs them nothing to re-test 30+ people using a technique they have already developed. The company will probably spend more on toilet paper this year.
They are doing an Aclaris, restricting the tests to people only in the norwood 3/4 scale, stacking the deck in their favour.
And limiting the classification to the vertex* (the trial calls for up to 6v) — few companies seem to have any interest in studying anterior Norwoods.
I really doubt that the goals of small companies such as Aclaris, Histogen and Replicel that get crucial funding or stock price increases after small encouraging updates can compare to the behemoth Shiseido’s goals.
Hey guys! It’s been 2 years now since I’ve been on this site. I ended up getting diagnosed with stage 4 colon cancer back in October of 2018. What a ride it’s been. I was supposed to be dead already but luckily some cutting edge science and great Dr’s at Memorial Sloan Kettering in NewYork were able to get me to remission. I just wanted to swing in and see if I could celebrate with a full head of hair yet- lol. It’s amazing the perspective that staring at your own mortality can give you about what really matters in life. Anyways, I hope every one is well.
Wow, very glad to hear you are doing better Erich.
What was your age when you were diagnosed?
I had just turned 40 the month before I was diagnosed.
Wow, that is crazy young for a colon cancer diagnosis.
Yeah, it’s on the rise big time in younger people these days. Doctors don’t know why.
I’m the first person in either side of my family to get any kind of cancer so it’s not hereditary.
Mine metastasized to my liver. I had 23 tumors there. I got this device called an HAI pump that is implanted in my abdomen that puts chemo straight to my liver at about 200 times the strength of regular systemic chemo, which I also did. That got me operable and they were able to cut out half of my liver and a foot of my colon. Luckily I didn’t have to have a colostomy bag. I lost a lot of my remaining hair on chemo but believe it or not it grew back a little thicker then it was before.
Wow what a crazy experience. If you ever want to write a post on how battling cancer affected your desire for hair (I am guessing still there, but not as much?) let me know and I would be happy to publish. Do you still take any hair loss medications?
I have had two family members and friends who had Chemo and both said their hair became thicker afterwards.
Cancer treatment has also shown to spectacularly reverse grey hair in some people:
https://twitter.com/JAMADerm/status/885257464702173184
Erich it is good to read Your post, wish You alll the best:)
Thanks Jan!
Erich i’m sorry about your stage 4 colon cancer. i’m really glad you’re in remission that’s great!
Thank you!
Erick thanks for sharing. I wish you continued success with your war on cancer:).
what worries me is the fact that in almost 25 years they have not discovered anything really new … By nature I am an optimistic person but this baldness crap has managed to erode all my optimism.
I think my question earlier came across as snarky but I was being sincere – am I completely naive if I hope that multiple injections, etc. might lead to a significant improvement?
Guys if any new treatment realy works it does not deamand multiple clinical studies. Thay could test it on one person and made conclusion. I am sure that the study results will claim something like that “intervention group had a significant increase in both hair count and follicle diameter about 5-15 percents”, but we have seen such treatments with “significant” regrowth for decades and none of the them have given visible results.
Clearly you are well-versed in science. Indeed, valid studies involve only one subject, the rest are obvious frauds.
Exactly. There is also no such thing as bias. It’s a myth.
You do not need to be well versed in science to understand that there are already a lot of such studies with zero sense. Yes, it’s better for me to see a cure that would actually help at least one person with advance AGA. Well, okay, not on one person, but on a small group of up to 10 people. We all know that the causes of AGA are the same for all people. By the way, this approach does not require large expenses. And the assessment of efficiency should be given not in percents of new hairs, but in photographs before and after, because there should be a visible result. If the effect is impressive, of course, it is necessary to conduct large-scale clinical trials according to all the rules, but if this is not the case, then it is better to spend time and resources on other reseachers.
It’s really weird and I don’t know how to interprete this trial launch from shiseido. My first thought is what a waste of time and money since replicel itself proved this product not to be significantly effective from an esthetic point of view. Plus, theres the situation with unsolved contractual issues with replicel, which doesn’t make things easier. So why launching trials now under consideration of difficult financial times?
Money is the least of problems of Shiseido.
Otherwise I completely agree with you.
RepliCel Terminates License Agreement with Shiseido
https://replicel.com/news/replicel-terminates-license-agreement-with-shiseido
I am not sure what this means, but thought it may be of relevance to this topic
https://www.replicel.com/news/replicel-provides-corporate-update
Thanks for posting admin, the devil’s in the details and the details of that report show that no real resolution of the Replicel dispute are expected until well into 2024. Disappointing.