Shiseido’s Dermal Sheath Cup Cell Hair Multiplication Treatment Has Arrived
Today might be among the five most important days in the hair loss world since I first started writing this blog in 2013. Shiseido’s decade-plus-long much awaited dermal sheath cup (DSC) cell hair multiplication (cell culturing and implantation) procedure is finally here for patients starting July 1, 2024. Albeit only in Japan, where they have favorable regulations for faster in-clinic use when it comes to regenerative medicine and stem cell based technologies.
Shiseido (Japan) completed small-scale Phase 3 equivalent trials for this hair multiplication technology in 2023. The results were positive but modest, with no major side effects. I hope the results will improve as they use this autologous DSC cell transplantation process in more patients and gain experience.
For now, you should only expect existing scalp hair thickening and perhaps minimal hair regrowth if you are lucky. Thicker hair in and of itself could lead to a slowdown in further damage from the ravages of dihydrotestosterone (DHT).
You can read today’s full announcement here (h/t “Theo” for the super find). The technology is described as having been developed by Tokyo Medical University Hospital, Kyorin University Hospital, and Shiseido. Interestingly, they leave out any mention of Replicel (Canada), with which Shiseido has had past legal issues after a technology sharing agreement went wrong.
S-DSC® Hair Regenerative Medicine
Shiseido has also created a new website related to S-DSC hair regenerative medicine, where the “S” stands for Shiseido. The about S-DSC page is very interesting. S-DSC® treatment promotes hair growth by supplementing the thinning areas of your scalp with your own cultured DSC cells.
They only make claims that existing hair will get thicker and more voluminous, while scalp inflammation will be reduced. Perhaps they also want to temper expectations, even if some people may get good regrowth? Interestingly, women might get better results then men.

The key person who led the development of this technology is Professor Emeritus (Department of Dermatology, Tokyo Medical University) Dr. Ryoji Tsuboi. I feel like a genius for using his image as my main one on my global hair loss research page from the get go. I originally picked Dr. Tsuboi over Dr. Takashi Tsuji just due to a more interesting image of the former at a white board.
Hair Regenerative Outpatient Treatment Begins in Japan on July 1, 2024
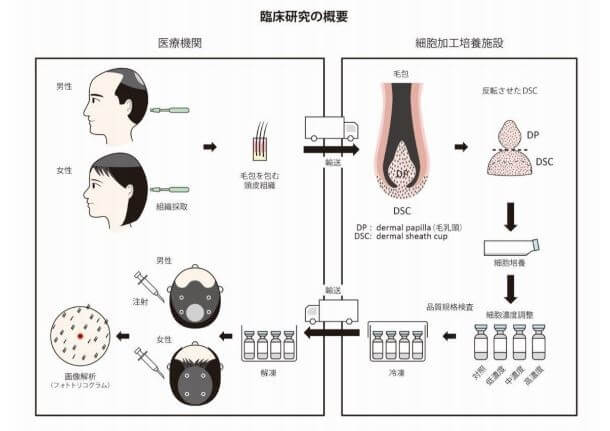
Associate Professor Shiro Niyama of the Department of Dermatology at Toho University Ohashi Hospital will start offering this “hair regenerative treatment for thinning hair” using cultured autologous hair bulb root sheath cell products (S-DSC®) from July 1st 2024. Patients will be required to get a referral, and also be responsible for their own costs due to the elective nature of the procedure. Note that this treatment is available for both men and women.
In the procedure itself:
- First, healthy scalp tissue of about 5 mm diameter is taken from a non-balding area such as the back of the head.
- From this, DSC cells are isolated.
- S-DSC is produced after about 6 weeks of culture and quality inspection (a total of 7 vials of S-DSC can be produced).
- Once production is completed, patients will return to the hospital and the cultured DSC cells will be injected with a special syringe.
Also check out the November 2023 study from the same team that is titled: “High migratory activity of dermal sheath cup cells associated with the clinical efficacy of autologous cell-based therapy for pattern hair loss.” The conclusion was that ITGA6-positive DSC cells, with superior migratory activity, may promote cell migration into nearby hair follicles.