Update July 26, 2024
KX-826 Hair Growth Spray Launched.
Kintor Pharmaceutical: KX-826 and GT20029 for Androgenetic Alopecia
My original post on Kintor Pharmaceutical (China) has become way too lengthy after so many updates. Therefore I will add all new developments in this post (below this product introduction section).
This rapidly moving well funded company is in Phase 2 and Phase 3 trials in the US and China for both its male androgenetic alopecia (AGA) products. Kintor (pipeline here) is conducting hair loss trials for:
- Two separate androgen receptor (AR) targeting products: a degrader (GT20029) and an antagonist (KX-826 aka Pyrilutamide).
- Each of these trials is being conducted in both China and the US.
- Each of these products is being tested for both males and females with androgenetic alopecia (aka pattern hair loss).
i.e., a total of 8 types of clinical trials times 3 phases in each = 24 developments we have to track. A bit too much if all of these do end up taking place, but a most welcome development. Over one-half of these 24 potential trials are already finished.
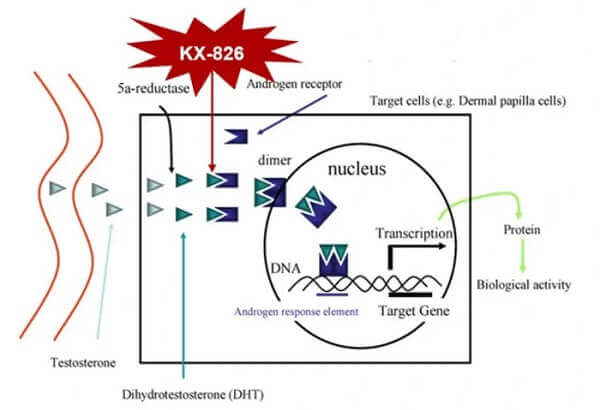
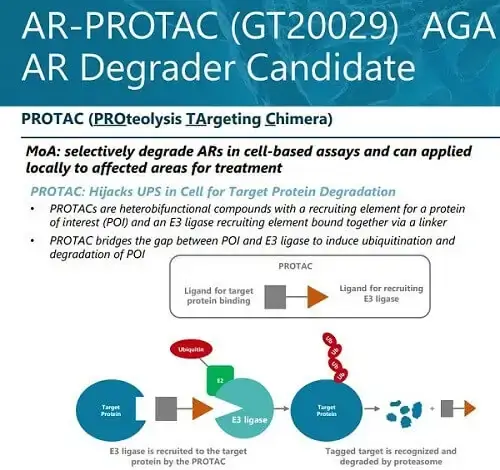
- The GT20029 product is an androgen receptor degrader (AR Degrader). It is developed using Kintor’s proprietary Proteolysis Targeting Chimera (PROTAC) platform. This is the world’s first topical androgen receptor (AR) compound (AR-PROTAC) to enter clinical trials. GT20029 degrades the AR protein via the E3 ubiquitin ligase pathway. During preclinical studies, GT20029 did not cause any notable side effects or systemic drug accumulation.
- Kintor’s main product for treating male pattern hair loss is KX-826 (Pyrilutamide) and is an androgen receptor antagonist (AR Antagonist). KX-826 is currently in Phase 3 clinical trials in both China and the US per the pipeline chart. In China, these trials are now completed.
Note that Cassiopea’s Breezula (Clascoterone) is an AR antagonist that is also currently in Phase 3 trials in the US. Kintor’s website has an interesting article discussing both these competing AR antagonist products and hair loss.
Make sure to also read my related past post on destroying the androgen receptor to reverse hair loss.
Update: July 11, 2024
Kintor Launches Cosmetics with KX-826 as the Main Ingredient
Kintor Pharmaceutical has officially launched a line of cosmetics with KX-826 as the main ingredient. This is the second widely discussed hair loss product that has been launched in the past two years as a cosmeceutical (with the first being CosmeRNA).
Update: June 5, 2024
KX-826 Receives The INCI Cosmetic Designation
Kintor Pharmaceutical’s KX-826 (Pyrilutamide) has received the International Nomenclature Cosmetic Ingredient (INCI) approval from the International Cosmetic Ingredient Nomenclature Committee. The assigned INCI name is Methylpyridinyl Fluoromethoxybenzonitrile Dimethyloxothiooxoimidazolidine. INCI names are recognized worldwide when identifying cosmetic ingredients. This development will facilitate the potential future global launch of Kintor’s line of cosmetics that have KX-826 as the main ingredient.
Update: May 24, 2024
More Effective KX-826 Tincture 1.0% versus 0.5%
Kintor’s clinical trial of KX-826 tincture 1.0% for treating male androgenetic alopecia in China just received clearance. The company’s pre-clinical studies showed that KX-826 tincture 1.0% had a significantly higher retention concentration level on human scalp cells compared to KX-826 tincture 0.5%. The latter was used in the Kintor’s previous phase III clinical trial.
Update: April 22, 2024
Positive Update on GT20029 Phase 2 Clinical Trial
Kintor Pharmaceutical just announced that its China Phase II clinical trial of its first-in-class androgen receptor (AR) proteolysis targeting chimera (PROTAC) compound GT20029 for treating androgenetic alopecia has reached the primary endpoint. The results are statistically significant and clinically meaningful, while safety and tolerability is good.
GT20029 demonstrated statistically significant efficacy compared to placebo in both the QD (once a day) and BIW (twice a week) dosing cohorts.
- After 12 weeks of treatment, the 0.5% QD GT20029 group showed an increase of 16.80 hairs/cm² from baseline. This was 6.69 hairs/cm² more than the placebo group.
- The total area hair count (TAHC) of GT20029 1.0% BIW group showed an increase of 11.94 hairs/cm² from baseline. This was 7.36 hairs/cm² more than the placebo.
- The 1% BIW dosage of GT20029 was identified as the optimal dosing level and has been recommended for the phase III clinical trial in China.
Key Quote:
“Based on the results of the Phase II clinical trial, the company will actively deploy subsequent clinical strategies for GT20029, such as initiating a phase III clinical trial in China and a phase II clinical trial in the US for male AGA.”
Update: February 1, 2024
Clinical Trial of KX-826 and Minoxidil
Kintor Pharma is undertaking clinical trials for two different androgen receptor targeting hair loss products (KX-826 and GT20029); in two countries (US and China); and for both males and females.
Now comes news that Kintor will also undertake Phase 1b/III clinical trials of KX-826 in combination with minoxidil for the treatment of androgenetic alopecia (AGA). The trial was just cleared by the National Medical Products Administration in China.
Update: November 27, 2023
Maybe Bad News for KX-826 Phase 3 Trial Results
According to this Reddit source, the results of the Chinese Phase 3 trials for KX-826 (aka Pyrilutamide) were ok, but not good enough. There were no serious adverse events or side effects. And positive hair growth was observed when compared to baseline (at all visit points) after 24 weeks. However, compared with the placebo group, the total area hair count (TAHC) improvement in the KX-826 treated group had no statistical significance. Albeit there was a “trend in efficacy observed”.
On a positive note, Kintor plans to continue with its multiple KX-826 related trials (in the US and China) for the topical treatment of androgenetic alopecia. I appreciate the speed with which the company released these findings. I expect there will be an official press release in the next few days. Edit: Seems like this never happened?
November 18, 2023
Phase 3 Trials for KX-826 (Pyrilutamide)
KX-826 (Pyrilutamide) is currently in the most advanced stage and has a good chance of coming to market in China by the end of 2024. In Kintor’s pipeline page, it is shown to be in the final stage of Phase 3 trials for men in China; and early stage of Phase 3 trials for men in the US. Moreover, it is also in the early stage of Phase 3 trials for women in China.
Kintor is also undertaking a second Phase 3 trial in China for long-term safety of KX-826, which I discussed in detail in my original post. Even more exciting, one my Chinese readers sent me the below partial translation of a new October 2023 presentation by Kintor. He said that his English is not good, but I only changed a few words that were confusing or out of place.
“KX-826 is a topical androgen receptor (AR) antagonist independently developed by Pioneer Pharmaceutical. It is the first AR antagonist in the world to enter phase III clinical trials for the treatment of hair loss. KX-826 binds the AR by competing with dihydrotestosterone (DHT) to locally block androgen-mediated signaling to limit hair follicle miniaturization and promote peripheral hair growth. After reaching the circulatory system, KX-826 is rapidly metabolized into inactivated metabolites, which has little influence on the whole body AR signaling pathway and has good safety.
The Phase II clinical trial in China included 120 men with hair loss. Of these, 90 subjects were randomly assigned to KX-826 0.25% twice daily, KX-826 0.5% once daily, and KX-826 0.5%, and the remaining 30 subjects were randomly assigned to placebo. After 24 weeks of treatment, the 0.5% concentration group of KX-826 showed a significant improvement in the amount of non-vellus hair in the target area. An increase of 22.73 roots per square centimeter compared to baseline.
Finally, news about Phase III: KX-826 is currently in or planned for five Phase III clinical trials (two in China and three in the United States). Among them, the Chinese Phase III clinical trial of KX-826 for male alopecia has completed the last subject visit, and the company is making full efforts to promote the data collation, library un-blinding and data statistical analysis of this clinical trial.”
Phase 2 Trials for GT2009
In Kintor’s August 22, 2023 update, they announced the completion of patient enrollment in Phase II clinical trials of GT20029 for male pattern hair loss in China. The current pipeline shows that these trials are almost over in China, and close to beginning in the US.
In the middle the post, I have linked to the October 2023 Chinese presentation image that someone from China sent me. This Chinese correspondent attempted to translate it, but feel free to check for any errors if you know Chinese.
Great work, admin. I’m sure this took quite a bit of time. I appreciate it. I guess we’ll see how it goes from here.
Nothing will happen.
You’re probably didn’t and honestly, from what I’ve seen leaked (if true and could be way off) much of this is as good as minox. I’m getting a little tired of stuff that claims it’s as good as minox. We need better. Way better.
I ordered Pyrilutamide on the grey market, and got some pretty shite sides. I’m hoping the real deal won’t feel like that, as I can’t handle fin.
So mind your own business then.
Note that beezula is a mild fail since it didnt work longterm in the 12months study. The efficacy decreased significantly indiecating that this trend will continue over a year and patients end up under baseline nevertheless.
So breezula is trash and kx need to shownit works long term.
breezula loos 50% of its power after 6 months of usage…but that doesn’t mean that it does not work at all..i use it since 3 years..
kx is trash, used it for 8 month, no result at all…
Admin – are you expecting KX-826 to be available in the US market by end of 2024? Or just Chinese?
Great question.
Just Chinese approval (best case scenario, assuming Phase 3 results are good). But it is so easy to get drugs from China these days.
Btw Dutasteride is still only approved in South Korea and Japan for hair loss.
Why would someone use this vs fin/dut? As a stack? What’s the difference between an androgen blocker, degrader, and antagonist?
Like others have said, if it’s as good a minoxidil and finasteride, then I see no reason why would you change drugs. Let alone add more to the inconvenience of topical application rather than oral.
Given how strong androgenetic alopecia is we need all of them. Stopping the convertion T/DHT (Dutasteride/Fin) is the most critical step but blocking (KX-826) and degrading the AR (GT20029) will be crucial for achieving long term success (20+ years).
That approach will be a true game changer for the young starting early enough.
Probably just for people who can’t handle Fin or Dut.
I’m more interested in the AR degrader product. If it works as it’s theoretically claimed, the results could be very significant. Rigorous safety studies are essential though !
https://www.wionews.com/science/scientists-achieve-major-leap-forward-to-potential-hair-loss-cure-660474
Wow, now that is huge. Long term, of course (maybe not in my lifetime) , but amazing nonetheless.
100% I agree with you, minimum 40 years (if we’re being generous).
What’s the point then. Why care if there isn’t a cure coming soon?
You must be new in the forum ;-)
I was being cynical Alex.
Does anyone have any information from stemson therapeutics? What are they doing? It’s been awhile.
Hope you have a good thanksgiving admin and everyone. (if you live in the U.S) and Yoda :). Take care.
Thanks and you too!
kintor released new news today, kx826 is hopeless, and its stock price has plummeted. I couldn’t cry or laugh when I heard the news. I was numb.
Thanks. There is some discussion about this on Reddit, but nothing on their site.
Sounds bad, though at least they released the results super fast.
https://www.reddit.com/r/tressless/comments/184pdh7/kintor_has_released_an_investor_relations_memo/
The last hope for a short term new product is breezula… After that, no new meaningful treatment until… 2030.
Kintor released the press release overnight (US time).
https://www.marketscreener.com/quote/stock/KINTOR-PHARMACEUTICAL-LIM-111325374/news/Kintor-Pharmaceutical-Limited-Announces-Results-of-Phase-III-Clinical-Trial-of-KX-826-Topline-Treatm-45442274/
I initially had high hopes for them until the Phase II results disappointed. I wish they would just drop KX826 and focus all their hairloss energies and resources on GT20029.
Thanks. They have enough resources, so hopefully no cannibalization of time and staff across so many trials.
Tough day.
Well I still can’t see official news on their website, but one has to assume the information is legit. I was wrong and initially thought the posts are doomerism and pessimism yet again…well alright.
Here we are, the one possible future product with the least efficacy (see phase 2) is gone down the drain. I personally wouldn’t have bought it anyhow, application twice daily for a dozen of hair is not worth it for me.
It makes no difference guys. We need treatments which are better than anything on the market now. Looking forward to Q1 when they release GT-results. I also think the company needs a homerun, there’s too many failed products already (see Covid-medicine) – otherwise they’re gone.
Make sense Ben. I deleted your short comment (as you probably guessed), but I would have thought the same in your place.
I had the advantage of being able to see that reader’s email address (a Chinese one) and his past one other comment on this site, so decided to check Reddit before concluding anything.
Totally fine with the deletion as I was in the wrong.
There’s been these absolute pessimistic and annoying comments lately from the known trolls which I want to counter (sometimes), and I shot from the hip in this case.
Let’s try to keep an optimistic view, as hard as it is sometimes.
This completely explains why they started the long term 52 week phase 3 trial this past summer. They saw that the efficacy wasn’t great but trending in the right direction, and they’re hoping a longer trial period will show a clear divergence in results vs placebo.
This also explains how breezula has designed their phase 3 trials, as they’ve had similar issues with their results.
The overall consensus on topical androgen antagonists is that they work, but not as good as 5a inhibitors. Therefore, they’ll need more time to show results which I think is worth it. This could still replace 5a inhibitors or stacked with it for more total efficacy.
I hope they commercialize this asap, and not wait for their long term phase 3 study.
Great point Johnny, and hope that is the reason!
Good post. Hope youre right as we need more weapons to the arsenal. AGA is a pretty strong beast.
No, breezula actually lost efficacy after 6 months with a decline to 9 months and was barley above basline at 12 months. When this trend continues breezula is trash.
For kx i dont know how 3 trials male and females has showed efficacy and now all of a sudden the point changed. Its interesting neverless if the product is trash or the trial design or maybe even the data analisis.
Phase 2 gt20029 is a pivotal moment for the company otherwise it may say good bye.
Yes, we’re aware of breezula’s declining results for months 9 and 12. If you look at their phase 3 design, they’re taking only those that were on the actual drug after 6 months and testing them vs placebo for an additional 6 months. It seems that their goal is to show that even though the results don’t hold long term, it’s still gives you more hair than you would have without the drug.
Not every new hair loss drug is going to be as efficacious as finasteride. It’s a different MOA that will show clear benefits and will appeal to way more people because of its 0 side effect profile.
I had a feeling that Pyrilutamide wouldn’t be as amazing as advertised. Especially at this point since 1993, big interventions are needed for drastic improvement of hair, dermal papilla multiplication, hair cloning, Amplifica, GT20029 etc
Im really lost as to how the results can suddenly drop off a cliff…anyone?
Are we saying it’s a total dud?
That’s the thing. Phase 2 data showed this was comparable to finasteride. We haven’t seen the data yet for phase 3, but now they’re saying that it worked but it wasn’t much better than placebo.
Hard to draw any conclusions until they share the actual data and we can compare them to phase 2 results.
Is it fair to say that it’s also unlikely to be effective for maintenance?
I don’t think that’s fair to say at all. Without seeing the actual data, we’re just guessing. But if that memo is legit, it sounds like the company still believes in the efficacy of this drug, and that they’ll be able to show it with longer 52 week study, which they’ve already started.
But even based on this memo, it said they’ve observed positive hair counts versus baseline over 6 months. Most people who are balding and not taking any treatments for hair loss will gradually continue to lose hair or stay the same after 6 months. Rarely do they see increases in hair counts, so this drug seems to not only maintain hair but also regrow some.
It’s the under performing delta versus placebo that’s confusing.
I am not surprised at all. We need something that shows legit hair growth. Not anti androgen topicals etc. On to the next drug. Hopefully that Bayer drug with the monkeys will work….2040 will have cloning…in the meantime it’s the big 3 snd fue.
I get legit hair growth from Pyrilutamide. I have been on it for over a year and basically regrew my bald temples.
I saw that three netizens in the stock market called kintor, and they were the following responses: 1. “The other party said that there was a mistake in the operation calculation, and they will continue to find the cause and strive for listing next year” 2. “I just called and asked, and I was analyzing the data, but they all think that the result is unlikely to reverse, and there will be no further announcements.” 3. “Just hit, the possibility of re-listing is not big, basically declared failure”
alas!
what is a netizens?
I’m open to anything but when they say “in combination with min” they lose me. That means by itself it stinks. And we already know minox isn’t a cure (I’ve been on it for months – stopped shedding but it’s not a real game changer, though yes it’s helpful). I feel like this is another bust, unfortunately.
The combo w/Minox seems like throwing spaghetti at the wall, Hail Mary to get some assemblense of results. I’ll take it if it’s an improvement of effectiveness over what we have now. If not, what’s the point?
Thanks for this update, we’ve all been waiting with bated breath to hear about GT20029. But given that Pyrilutamide also seemed promising after phase 2 trials, how skeptical should we be here?
During the last 15 years, how many phase 2 trials results are statistically significant and clinically meaningful, yet failed to reach phase 3 or market ? I lost the count.
Agree… I’m still waiting on word from the Maksim Plikus trials. He sure had a ton of confidence but no news so far!
About half-a-dozen. The greatest disappointment was Follica, because that protocol works and many people are getting great results with a crude, home-brewed version of Follica’s optimized technology. The admin should change the name of this site to HLC2050, because that’s probably an accurate prediction.
I was certain that Follica would be commercially available by 2023 (my “worst case” scenario was by Q2 ’24); at this point, all men should be subscribed Finasteride prophylactically on their 22nd birthday – it’s really the only solution to this problem.
Finasteride looses it’s effectiveness over time, all treatments do to one degree or another. Or maybe our baldness genes become more potent. I know, started (topical) minox approximately 40 years ago, (oral) fin (now dut) 30 years years ago. Need to keep tinkering with oral, compounded topical versions in the attempt to tread water. I’m grateful to have at least had these tools in the toolbox, however I sure as hell hope some guy isn’t writing something similar 30-40 years from now! :-(
I agree, though the Japanese seem different:
https://www.hairlosscure2020.com/does-finasteride-work-after-20-years/
Right, my experience doesn’t mean everyone will be the same. Depends on your genes, a guy who has a lower degree of hair loss might fair better, e.g. Aston Kutcher. However for the majority that read this thread my guess is that the grim reaper of hair loss will always be after you. I’ve saved a lot of hair, however it’s not the same as 30 years ago, for sure.
Maybe it’s me but ‘statistically significant’ sounds impressive after just 12 weeks/3 months.
Normally with hairloss cures we tell our self’s to wait 6-8 months before judging.
Reddit discussion on the GT20029 China phase II trial primary endpoint results:
https://www.reddit.com/r/tressless/comments/1c9qdi6/gt20029_china_phase_ii_trial_for_aga_reached/
So Kintor’s GT20029 Phase II trial results were released yesterday, below are the current results of total Target Area Hair Count (TAHC) increase:
Finasteride:
Finasteride 1 mg/day significantly increase total hair count compared to placebo 12.4 hairs/cm2 after 24 weeks.
5% Minoxidil:
The mean change in nonvellus TAHC between baseline and week 16 was +22.0 hairs/cm2 (95% CI: 18.1; 25.9) in the DC0120 group and +20.5 hairs/cm2 (95% CI: 16.6; 24.4) in the comparator group.
Bioneer CosmeRNA:
The mean change and rate of total hair count increased by 7.545 ± 7.896 and 4.264% (p < 0.001) at 16 weeks and 7.727 ± 8.659 and 4.421% (p < 0.001) at 24 weeks in the AR68 5 mg/ml treatment group.
Technoderma (TDM-105795), Phase 2a trial:
The results indicated that TDM-105795 led to a mean increase in non-vellus Target Area Hair Count (TAHC) of 24.3 hairs/cm² with the high label strength (0.02%)
Kintor (GT20029), Phase II trial:
The TAHC of GT20029 1.0% BIW group showed an increase of 11.94 hairs/cm² from baseline, which was 7.36 hairs/cm² more than the placebo, also yielding statistically significant results (P<0.05) after 12 weeks.
Hope (HMI-115), Phase Ib trial:
14 hairs/cm² mean increase in non-vellus target area hair count (TAHC).
Breezula (CB-03-01), Phase II trial:
14.3 hairs/cm² increase after 12 months of 7.5% BID clascoterone solution dosage.
Follicum (FOL-005), Phase 2A trial:
The topical compound resulted in a 6.6 hair/cm² increase in hair growth.
Amplifica AMP-303 trial:
no results published yet, they announced that results should be out 2024Q1 but nothing so far.
Amplifica didn’t say that results should be out 2024 Q1. They said the trial would be completed by Q1, meaning that they require a few months minimum to gather the data, present it to FDA etc. The results won’t come out most probably before June.
Helpful post, is it just me or do most of these look like total sh*t for regrowth except technoderma? I hadn’t realised the pipeline was quite this lackluster.
It really is all about where you are in your hair loss stage. For those at NW 1-2, anything that even maintains for 10 years is amazing. For those at Norwood 3 but whose hair loss started less than a few years ago, a whole bunch of these treatments could bring back recently lost hair.
Then we also have the Fukuda dermal papilla transplant and Aderans cell therapy treatments that could come to market within several years.
What are your expectations of Pelage and Amplifica (in terms of timeline and effectiveness)?
Pelage is supposed to start Phase 2 trials in mid-2024 and has raised the requisite funds.
Amplifica was supposed to have finished Phase 1 trials in the first quarter of 2024.
So both are still a few years away from market, assuming all goes well in future trials.
For sure, I hold high hopes for both these, but I’m more so keen for Fukuda (timeline and efficacy).
Are you sure the amplifica AMP-303 trial was Ph1? I thought the drug was already proven safe in man.
Am away from computer, but maybe you can check my Amplifica post in case there are more details?
What about hairClone in UK they will start there trials in UK any news from them?
What does that INCI mean? Will it launch as Cosmeceutical as well or is it still a prescription drug only?
Acronym is spelled out in the update:-)
Not sure, but my guess is that they will try cosmetic first, then maybe (high-dose?) prescription too.
Thanks Admin, I should have asked more precise. My question was will it come as Cosmeceutical OTC and what would be the timeline to expect? But good to hear they move forward with both products for AGA in the pipeline.
More discussions on the cosmetic route here:
https://www.reddit.com/r/tressless/comments/1d907lf/kintor_to_launch_pyrilutamide_as_a_cosmetic/
But they’re still going through full clinical trials even though it’s a cosmetic, right?
They have gone through Phase 2 and/or Phase 3 trials for both key products. They will likely be able to use them in cosmetics without the need for another 5 years of trials.
But Pyrilutamide failed Phase 3. How can anybody trust their products if they don’t demonstrate that the higher dose is effective?
It is not clear if it is a certain failure, since there was some hair growth.
They are continuing with Phase 3 trials for Pyrilutamide, and also check out the May 24 update on 1% versus 0.5% trials. But my gut feeling is that this will not be much better than Finasteride. Albeit with hopefully no side effects.
If it doesn’t work they can bring out as a cosmetic. It will perform as well as all cosmetic treatments for hair loss have to date (sarcasm)!
All my posts are not going through, admin. FYI.
Hey Ben, thanks for letting me know here and via email! For some reason, a lot of comments are going into the “Trash” folder (which I almost never check) rather than the “Spam” folder. I managed to get most of them moved over in time thankfully.
The spam control plugin that I use is also giving me some strange messages lately. Will try to resolve.
Do we know when the results for the phase 3 results for the 1% Pyrilutamide trials are expected?
Thanks for all of your hard work and for keeping us in formed.
Hi @Admin,
did you see: https://www.topadur.com/pipeline
Medical cosmetics|TOP-M119
Alopecia, Skin aging
Solution -> TOP-M119
Yeah it is still in pre-clinical. Someone asked me last year to write about them, but I will wait till they start Phase 1.
Stemson has laid off over 10 employees.
The twice weekly protocol for GT20029 is stupid! If the concentration of 0.5% is used daily, then it is reasonable to use the concentration of 1% every other day.
A question about pyrilutamide:
If 1% concentration is more effective (and safe), why didn’t they use this concentration from the beginning? What guarantee is there that the concentration determined for GT20029 is correct? This company is not careful in determining the maximum safe dose.
GT20029 group buy.
Admin: I removed the Discord link as I am not sure about these suppliers. You can find these links on Reddit or on the various hair loss Discords, if you want to risk it!
You can post such links on this site’s Discord chat FYI. I do not log in to that Discord daily, but email me for an invite if you want to join.
Hi Admin,
We want to risk it from here. Please share the details and leave the rest for us to decide. We will do out own research.
https://en.kintor.com.cn/news/258.html
Launched!
Where do you buy this?
Why would they launch it as a cosmetic since they’re doing clinical trials including phase 3 ? Will they sell it as two separate products in the future ? One cosmetic and one as medical treatment ?
The cosmetic is launched.
Admin: Thanks, just published a new post on that, but removed your link above and added my replacement text.