Thanks to readers “Ben” and “Theo” for the regular updates on Epibiotech (South Korea). Note that Stemore was renamed to Epibiotech in 2021. Since then, the new company has been in the news almost every month. Note that besides EPI-001, Epibiotech has several other hair loss products in its pipeline that I have listed at the bottom of this post. The top half is all recent updates.
Update: May 9, 2024
Epibiotech Receives Funding to Begin Clinical Trials
Epibiotech just received approval for government funding for its pending Phase 1/2a clinical trials. I expect these trials to begin before the end of 2024.
Per CEO Seong Jong-hyuk, this is the first clinical trial in the world to conduct an autologous cell therapy for hair loss using hair papilla cells. I am not sure if this is true, since Intercytex completed Phase 1 trials on its dermal papilla expansion and transplantation (vie injection) process several decades ago.
Moreover, the Fukuda Lab team in Japan recently announced that dermal papilla cell transplantation is about to begin in Japan. And perhaps Han Bio and HairClone have both further advanced their dermal papilla cell expansion related technology as of 2024.
New Epibiotech Video:
Update: April 23, 2023
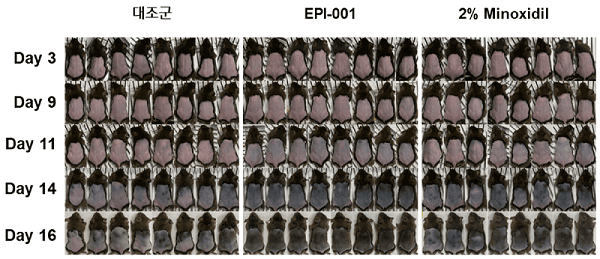
Epibiotech has succeeded in growing hair in pigs via the injection of human dermal papilla cells (hDPCs). I discuss the details of this significant development in my post on pigs and hair growth.
Update: February 9, 2023
New Epibiotech CEO Interview
A very detailed new interview with Epibiotech CEO Sung Jong-hyeok. The translated title states that a powerful new drug for the fundamental treatment of hair loss is coming. However, Phase 2 trials are yet to begin for their main EPI-001 product. Key quote and photo below. Note that they are working on several different ways to tackle hair loss.
“There are many companies worldwide that conduct research on hair loss. But we are confident that Epibiotech is the only company that is developing various modality treatments based on cells, antibodies, and low-molecular compounds (chemicals) and that is conducting the most in-depth research.”
“In addition to the currently leading dermal papilla cell-based new hair loss drug, it is discovering and developing new hair loss treatment targets such as CXCL12 neutralizing antibody and RIPK1 inhibitor.”
Update: January 20, 2023
Epibiotech and Novelty Nobility sign an agreement to develop an antibody treatment for hair loss. Epibiotech plans to add any new antibodies discovered by Novelty Nobility to its own hair loss treatment protocol.
Update: November 22, 2022
A new paper from the CEO on cell therapy for hair regeneration that necessitated my writing an entire blog post.
Update: September 9, 2022
Epibiotech and Humedix to Jointly Develop Hair Loss Treatment
Thanks to reader “Theo” for the below two recent updates. On September 7, Epibiotech and Humedix announced a new partnership. The two companies will collaborate to further develop Epibiotech’s hair loss treatment. Humedix seems to be a reputable cosmetics sector player, with significant experience in manufacturing injection and platform technology.
Moreover, according to the press release, Humedix is also developing:
“Spheroid culture technology through 3D culture of dermal papilla cells and a scaffold using biopolymer materials such as bio-ink related. The goal is to expand the business into the cell therapy field by combining 3D bioprinting technology and biopolymer application technology.”
In the article, Epibiotech is described as having expertise in four key areas:
- Hair papilla cell isolation and cultivation technology.
- Induced pluripotent stem cell manufacturing technology.
- Organoid-based hair follicle cell differentiation technology.
- Gene editing technology.
Also of interest, in August 2022 Epibiotech hired two new staff members to its leadership team. One of them is described as a vice president of the Ministry of Food and Drug Safety. He is an expert in the medical product field and a regulatory approval review specialist.
Other Recent Updates
Epibiotech CEO Sung Jong-hyeok seems to give numerous documented presentations at local conferences, universities and more. Some recent highlights:
- Update: July 12, 2022 — Epibotech human clinical trials to commence with a year. Another article from earlier this month states that a dermal papilla cell pipeline, was completed in June 2022. The company “will apply for an IND in the second half of the year and enter Phase 1 clinical trials in 2023″. Also of note, preclinical work confirmed very good efficacy. One month after transplantation of human dermal papilla cells into pig skin, the number of hairs increased by 40% and the hair thickness by 30%.
- Update: April 29, 2022 — Epibiotech announced on April 29, 2022 that it would expand the EPI-001 autologous DPC (dermal hair papilla cell) therapy to off-the-shelf allogeneic treatment.
- Update: April 21, 2022 — Scientists from Epi Biotech were involved in an interesting new paper. Its conclusion: “Silencing the CXCL12/CXCR4 signaling pathway can be developed for novel hair growth stimulation.”
- Update: October 5, 2021 — Epibiotech has started producing non-clinical samples of its dermal papilla cell therapy EPI-001 at its cell therapy production center.
Epibiotech New Hair Loss Facility
- On June 3rd 2021, Epibiotech and T&R Biotech announced a partnership to develop a hair loss treatment involving pluripotent stem cells. According to T&R Biotech’s CEO:
“Epibiotech’s dermal papilla cell differentiation technology and our pluripotent stem cell line will create a combination with high synergetic effect.”
- On May 31, 2021, Epibiotech and its CEO Jong-Hyuk Sung announced the construction of a new production facility at its Songdo headquarters. This GMP facility will be used to produce “2,000 self-made dermal papilla cell therapy products annually”.
Epibiotech’s platform technology page is all over the place and a bit confusing. It includes dermal papilla cell separation and culture technology as well as gene editing based hair follicle stem cell production.
Note that CEO Jong Hyuk Sung is a professor at Yonsei University. This company was originally founded in 2015.
- On April 30, 2021, Epibiotech signed an agreement with Professor Hyung-beom Kim of Yonsei University to develop a hair loss treatment using gene editing technology.
All of this seems a bit too fast and crazy.
However, as I outlined in my recent post on Han Bio, South Korea is becoming a world center for hair loss cure research. On par with the US and Japan if media articles are to be believed.
Renaming from Stemore
I e-mailed Epibiotech to inquire about their technology and their May 1, 2021 renaming from Stemore. Below is part of the reply (with minor corrections) from Dr. Nahyun Choi, the company’s director of research:
“The name STEMORE, used previously, was based on a focus on research on hair loss using stem cells when the company was founded. However, our company is now researching and developing not only stem cells, but also various hair cells. This includes dermal papilla cells, and an expanded pipeline to low molecular substances and whitening agents. Therefore, the scope of the research and development was extended to hair and skin. So the company name was changed to Epibiotech after the foreword of epidermal cells.”
Epibiotech Pipeline
According to the company’s pipeline page, they are working on three hair loss products.
- The main focus is on EPI-001, the autologous dermal papilla hair cell therapy treatment (with paracrine effect). It is scheduled to enter Phase 2 clinical trials in 2024. They have added EPI-008 in this category too.
- The EPI-002 RIPK1 inhibiter (gel or ointment) will also enter Phase 3 trials in 2024.
- EPI-005 CXCL12 inhibitor.
- EPI-007 Wnt signaling modulator (with paracrine effect). Seems to now have disappeared.
- They previously also showed EPI-003, an oral PGD2 inhibitor product. It was supposed to finish Phase 2 clinical trials in early 2025. Now it seems to be have been removed from the above list.
I hope this isn’t another Replicel.
Yeah so are they working on hair cloning? Or just topical? If its just a topical I honestly don’t think it will help much.
Hair multiplication.
The next treatment is expected to be released in the next 105 years
@yoyo thank you for the explanation, so they are working on hair cloning and not just a injectable? Or both? Thanks again.
They are working on three separate solutions for hair loss. The information on their website is very confusing and all over the place, but from what I understand, the 1st is hair multiplication by extracting a few hairs from the back of the patients head and culturing those hair follicles to mass produce and transplant them on the patient, like a regular hair transplant. However, they also mention stem cell research from iPSC (very similar to Stemson if you are aware of what they are currently doing). The 2nd is some ointment/gel that inhibits Ripk1, not too sure what that is. The 3rd being a Prostaglandin inhibitor which you take orally, there was research a few years ago that showed that prostaglandin can also contribute to hair loss so this medication is supposed to inhibit it. Being a med student I know that there can be some rare serious side effects inhibiting prostaglandin though, so I’m not sure on the safety of such a medication. If you want my honest opinion you should wait on Stemson, they are funded by Allergan which is a famous pharmaceutical company and the science and technology behind Stemson seems solid. They are currently testing their product on pigs (similar skin to humans, mice aren’t as reliable). Wait around the end of this year to see what their preclinical results will show, plus it’ll be way more affordable than Tsuji. Hope this helps.
@yoyo dude thank you very much for the explanation I was confused about it. OK so wait for stemsons therapeutic which might be available in 2030 just kidding but remember that link Jan sent us on here where fortunis there investment partner said the pig trials where a success and that they were hoping to start human trials later this year oh by the way they took that video off of vimeo I can’t find it anywhere anymore. But anyways we now have 5 companies working on hair cloning and I really only trust stemsons therapeutics. I honestly feel that Dr tsuji is done and over with and tissuse is a joke.
No worries dude. https://www.linkedin.com/jobs/stemson-therapeutics-jobs-worldwide/?f_C=20275102&trk=top-card_top-card-primary-button-top-card-primary-cta This website shows the job listings that Stemson are currently looking for, by the end of this year hopefully there should be an update on what progress has been made (and human trials next year hopefully). Alexey did say he expects this to come to the market around 2023/2024, but did say it would probably take a little longer than that. Yes Tissuse is nonsense, Tsuji is a big question mark for me, the price is ridiculous. Yokohoma, Epibiotech, Han Bio are all to look out for. There’s also GT20029 from Kintor which is an androgen degrader which I’m excited for but that might be a while.
Great research!
My man.
Hi admin,
Can somebody translate announcement on web page. Epibiotech has no English version.
It is great news if it is real.
More like they’re working on money multiplication via fleecing investors, like every other hair loss company for the last 50 years
I always find it flattering to be credited, thanks Admin.
Great article. The race is on, Yokohama vs Stemson vs Epibiotech in the cell-approach. Tsuji being a questionmark. Hairclone is out imho.
Yup I agree, hairclone, replicel, follicum are hot garbage.
Hairclone was always out lol in my opinion. They should just be a company that only preserves your follicles thats a good business model. It’s insurance for your hair.
what? come on it’s totally useless. this is just a company who rob money from st**** people. They don’t care about people losing their hairs , they care about their wallet. Even the higher norwood still has hairs for future hypothetical hair multiplication therapy ($$$$$). you even find single hair follicle at the back of your head to multiply for your future hairline. that being said other people with most severe hair loss condition like alopecia totalis, universalis have nothing to bank unhappily because they have **** genes and would need to attack their hairloss from another angle.
Why would Tsuji be a question mark? Seems their issues now are more administrative and financial than technical. I think it’s just a matter of time for them.
Few years I guess. I think Tsuji will probably be the first to have a working cure on the market.
tk are you joking? In the past years before covid the guy raised hopes on his trials being accomplished several times by 2020/21. When the time finally came, no announcement at all on the current state of trials was made, we didn’t even know at that time of they occurred at all. First and last thing announced was one of their main companies driving the technology went bankrupt, that’s all. Tsuji and related firms failed totally. Even replicel has higher rank since they had trials and announced their (unsuccessful) results.
@red exactly.
Seems like the race is really on. For the first time ever I feel kinda hopeful. Let the last of the real ones battle it out. They all have promise (those four or five). Let’s goooo!
Does anyone remember if the marketing of propecia was pre-announced in some medical magazine or other media? Or was it a sudden news?
Lorence I remember just seeing propecia as on tv when it came out in 1997.
Admin which brand on vitamin d do you take? I’m trying to get a liquid vitamin d the regular vitamins arent raising my levels.
See top paragraph:
https://www.hairlosscure2020.com/vitamin-d-and-hair-loss/
NOW Foods- Liquid Vitamin D-3 Extra Strength – I’ve been taking 10 000 UI/day for 8 years more or less (Bechet disease) For me the best value hands down, I tried every major brand. Fun fact, this hormone (yes it is) will help “regulate” your mood ps: Anything below 8 000UI didn’t help much.
And I wonder what the people that say hair cloning is years away or never coming are on these sites now, said about Finasteride back in the 80s or 90s when it wasn’t available until 1997 I wonder if they were always this negative?
I feel like people who were experiencing hair loss in the early 2000’s/90’s were expecting hair cloning or a cure back then. However, the science and technology weren’t even close to what it is now and over the years they developed immense negativity because they were promised hair cloning and etc back then so they create doubt in future generations who are currently experiencing hair loss. So people talk about Intercytex for example, back in 2006 where they tried hair cloning and failed because of poor financial support and underdeveloped science and technology. The past 5 years alone have taught so much about hair loss that we never knew before. So of course they hold onto this negativity and pass it to future generations to not get your hopes up. However, there is so much to look forward to now, for example, OliX, Aneira, Cassiopea, Kintor, Stemson, Epibiotech, Tsuji(?), Yokohama, Han Bio etc. Hair loss suffers have so much to look forward to. Let’s just stay hopeful and patient that a cure will be viable in the near future. Sorry for the long response lol.
Great comment YoYo.
If you are 20 and losing your hair, consider yourself more than lucky if a cure comes out in 10 years. If a cure would have come up when I was 30 by now I would have already lived almost 20 years of my life with a full head of hair, unfortunately it did not happen …
Great comment yoyo!
We do have many companies back then and including Dutasteride on pipeline and Ru….If all the companies you mention, one make it , we will lucky . Cassiopea is on clinical trial with different names since more than 10 years ago . Kintor clinical trial are made in China ! I will not put my money and get real info from there , could be simple a big scam .
Anyone know the reason why vitamin d levels can be extremely challenging to raise when one is on fin (for some people)? When I was on fin best I could do after heavy supplementation was to get my levels to the low normal range. Now that I’ve been off fin for years I take a vitamin every now and then and my levels are in good range.
Tom Jones I have the same problem. Low range fir me and cant get it higher
Thx admin…will check it
I’ve been following different forums and followed different kinds of stuff trough the years. From about 16 years of age until now. 36. To be honest. All I’ve seen is just plain bs. Did minox and fina for a long time. But now i give up. This s ain’t gonna be fixed when I’m still young so this stuff… Correct me if I’m wrong. But for me it just seems like old news changed name. It’s ok now. I can be Kojak.
Started following things here on HLC several years ago and occasionally over on FT. Both very informative sites. I don’t even bother with The Bald Transplant or YouTube anymore. I like YoYo’s comments but I remain cautiously sceptical, still hopeful in some ways, but not holding my breath either.
Reddit and HLT are also great. Have not been browsing the other hair loss forums much during the past several years.
https://www.hairlosscure2020.com/list-of-hair-loss-forums-around-the-world/
Exactly.
https://www.nature.com/articles/s41392-020-00441-y admin did you do a post on this? i can’t remember.
Do not recall, though article itself seems familiar.
They will never find a cure…All the big shampoo companies buy up all the patents…so they can make billions selling you things that don’t work.
Jom Smith had a valid point.
He has not, this is not how market works, otherwise there will never be new cars because they steal business to those who are already around. No new phone either.
Same with literally every technology developed by the human race that has sometimes killed a business (but sometimes created another one!). Market is based on competition.
Plus, an effective cure would attract much more customers than the lousy solutions we have today. May I remind you that the vast majority of bald people have probably never even seen a pill of finasteride and if they did they said no in fear of its side effects?
C’mon, stop with this childish conspiracy theories!
Truth.
The difference is these are research based products/solutions and thus the investment required are high risk (very few are profitable at all). Markets don’t incentivize such breakthroughs.
These kinds of breakthroughs tend to occur primarily in academic settings where risk is not a deterrent.
I’m not saying there’s a conspiracy but the free market isn’t highly incentivized to solve this problem.
So what does this update mean? Should I have a drink to celebrate?
I would say there are many questions open. Stemson develops an allogenic solution too, but for the whole follicle.
This seems to be „only“ a DP-cell-therapy, afaik – which has yet to be proven successful and effective.
Allogenic though is great – this would make the treatment fairly easy and probably cheaper: a one-way-trip to Korea. On the other side I expect a longer process in terms of time to market.
It seems that Epibiotech is very confident in their tech and they have a good number of employees and a solid funding. So far so good.
That’s what I’m tryna figure out what tf does this mean?
Hi Admin, your take on HUTERA? The data looks pretty amazing to me:
https://m.blog.naver.com/PostView.naver?blogId=moogene2&logNo=222718428536&navType=by
Summary:
• serum concentration is only 1 % of oral Dutasteride
• dermal delivery 20 times higher than oral dutasteride
• local scalp administration 1 – 2 times per week, done within 3 minutes
• commercialization in 2023
There’s also pictures of the product in the blog post. If this doesn’t work highly effective, than nothing does. HUTERA could be a new weapon in the near future.
Here’s the paper from 2020:
https://pubmed.ncbi.nlm.nih.gov/31901692/
Really interesting Ben. I will try to read more today and write about it if justified.
Super interesting Ben, if true it could be a game changer to have super high levels of Dutasteride in the scalp with almost none going systemic. I wonder if this procedure can be done at home or if you would need to go to a clinic?
As someone who can’t take Fin because of side effects, I’m excited about this.
Also, I read the paper abstract. They talk about using the micro bubble/nanoliposomal technology to deliver Crispr-Cas9, to knock out the gene for 5 alpha reductase in the scalp. The use of Crispr-Cas9 to edit genes in humans is in very early days, but if it becomes mainstream this would be very exciting. Basically the hair follicles could no longer produce the enzyme to make DHT.
I just wish I was 20 rather than 40 so this stuff would arrive in time to save my hair.
The Crispr-Cas9 part in the paper is a little confusing to me.
I am not sure, but to me it seems they use Dutasteride as a treatment, but the delivery is based on Crispr-Cas9 and nanoliposomal lipids? Maybe you can shed some light on this issue?
I am pretty sure though this is a at-home-treatment (see pictures of the product). You just cannot go to a clinic two times a week for a treatment duration of 3 minutes.
HI Ben,
Yes, from what I can gather, the dutasteride delivery system must be based on the microbubble/nanomicrosmal system used to deliver Crispr-Cas9 in the paper.
I know that several places have been working on these delivery systems for Crispr-Cas9 – without them, the guide RNAs would be rapidly degraded, and the Cripr-Cas9 probably wouldn’t work. The ultrasound activation is new to me though, pretty cool technology.
I’m so excited about this if it actually works. Having a robust, low side effect treatment available next year would be a game changer for those of us with some hair left.
Wait…increased hair by 40% and increased thickness by 30%? That’s off the charts good. And it was a pig so maybe with a human even better. And maybe multiple times/injections would increase it even more.
I always thought this would be cured by something similar to this. There’s no way rubbing cream on your dome was ever going to cure baldness (just help keep what you have for a while).
This is great news. Fingers crossed it stays on track.
I agree, I didn‘t get much media cover though. There is no research paper to this available, hence maybe no reaction. If this can translate to humans, then it would be more or less a cure for the majority of AA – an early intervention with this could possibly prevent any further hairloss.
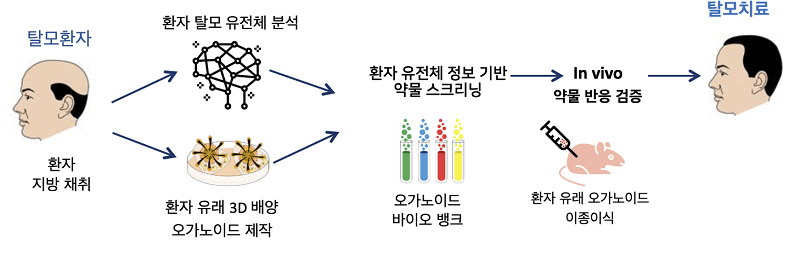
Epibiotech is my biggest hope, it’s the biggest hair-startup worldwide with 32 listed employees. They have 4 different arms atm, 2 cell treatments, 1 antibody, 1 Ripk-inhibitor. They will start the first cell-treatment for hairloss since Intercytex in 2010 (!).
They also research a „gene-edited“-therapy – it is its lead candidate „EPI-001“ combined with gene-altered DP-cells (i suspect it to be EPI-004). Here‘s the patent, it has fantastic results:
http://kpa.kipris.or.kr/kpa/biblioa.do?method=biblioFrame
Their early research als included olfactory receptors (see Ralf Paus), but apparently that was scrapped.
Humedix is sister company under ownership of very big pharma company in South Korea – Huons Global. So, they will have enough money to go through clinical trials.
@James1 40% increased hair after 1 month on pig skin. In 4-6 months results can improve significantly. That is my opinion.
@Bryan – thanks for the clarification on Humedix. That sounds really good.
The question is, as always, if results can be translated into humans.
The numbers after 1 month are insane. If multiple treatments work better, and how long the results last at all – nobody knows yet. But soon we will see.
I agree, it could be even better over time or repeating the process. Total game changer. Potentially, anyway. I’m a skeptic at heart. Time will tell. I try not to get too excited.
I finally got off the finasteride pills. I had been on finasteride or Avodart since 1997. Been using them for years with minoxidil. I switched to finasteride / minoxidil combo topical last week. This week I had a surge in libido and my weightlifting strength has taken off. I feel like I reversed my age in years. Coincidence? Possibly. But I’m only hoping I never have to get back on those damn finasteride pills.
I’m sorry to spoil your excitement, I did the same and after a year I had to go back to take oral finasteride. Why after a year? because after a year excessive hair loss started again and I doubt topical finasteride did anything. I hope you’ll have a better experience, let us know.
@lorence – I’m not expecting results to be better than with the pill – to the contrary, I’m just hoping the topical fin / minox combo aren’t that much worse. But I’m tinkering with the prescription formula a bit. If I have any good news, I’ll post. If all I get is my youthful sex drive back and my youthful strength back, like I’ve experienced this week, I’d be ecstatic.
Fin and oral minox just aren’t good for you. According to my new doc. He said they’re studying long term sides and he doesn’t recommend either for anyone. I tend to agree, based on my experience. Maybe it’s not as bad for some but maybe also the impacts won’t be realized until the 20 year mark. I don’t like either of these products, honestly. And we’ve all been stuck on them for way too long.
Any idea if how much this treatment will differ from Hairclones dermal papilla injections? Any word from Hairclone when theirs will finally be available? Was meant to be beginning of this year, then pushed to the summer, latest I heard was end of this year. Have you heard anything Admin?
No, just what is in here:
https://www.wired.com/story/new-baldness-treatments/
@Ben
Behind Epibiotech are professors and guys from Seoul University. Seoul University is 9th ranked in whole Asia and 60th in whole world, so these guys are no joke.
Pig skin is the closest to human skin, so this technology will work in humans for sure, but how good the results will be that only clinical human trials will tell us. Investments and collaboration with big pharma company and moving fast are telling us that they are pretty confident in what they are doing.
If this works then it will need to hit up 3 clinic trials 7 to 10 yrs. Can this be fast tracked?
It sounds like potential. I prefer injectable treatments at the source than topicals. Hopefully this and Bayer will be our new major breakthroughs.
I’ve been reading your comments for a while and idk where you get your extreme theories from. Did you not read that this is not going through the USA clinical trials, even if it were, it wouldn’t take a decade to be approved. How could the company support itself and continue to get investments with a return investment in 7-10 years?
Based on news Epibiotech and Humedix will exchange technologies, experience, science advisers and expand collaboration in very complex technologies like 3D/4D bioprinting or gene editing (CRISPR).
Humedix is sister company to very big pharma company Huons Global founded in 2002. Huons Global was founded in 1965 and they are big player in South Korea and worldwide.
YoYo I get my info from the FDA. I was referring to usa not abroad as I live in the USA. For me, I will need to wait for all three trials for it to be approved here. Yes, abroad would be quicker Hopefully. But most drugs and new cell treatments like this will need to go through in depth trials here in the USA. Hopefully you can get it sooner overseas.
I live in the States too but I’ve never heard 7 years of trials…
One can travel overseas to get the treatments, no? You don’t have to wait for the US to approve it.
It’s up to the company to figure out how they can fund 7 years of trials….thsts why most small companies don’t make it and these hair loss companies don’t get funding after two trials due to the fact the product doesn’t work…
YoYo, Mjones is right, in regards to your question “How could the company support itself and continue to get investments with a return investment in 7-10 years?”
This is true of any startup Biotech, they raise capital from investors to fund their activities until the drug/treatment is released, get acquired or they shutdown (due to not getting approved, lack of funds or poor clinical trial results). This can take 5,7,10 or more years easy. It happens across the spectrum of drug development.
Unless it’s injections I would need weekly or monthly, I would gladly fly to another country to get them. Quarterly? Probably. But once or twice a year – you betcha. Nobody has to wait for approval in the US. If you got the cash, hop on a plane. Make a holiday out of it. If there was a guaranteed cure in some other country there’s no way I would wait a decade for the American FDA to approve it. I’d go to where the cure is.
I should add that my privilege is showing – I completely understand for the majority this isn’t an option. But if it’s once a year (if we can be that lucky), and the procedure isn’t that costly, it’s worth saving up for that plane ticket and hotel (and honestly I bet the procedure is cheaper than it would be in the US anyway).
So correct me if I’m wrong but epibiotech is targeting human trials for hair cloning for next year they also have a topical solution and a pill for hair loss correct? Why do a topical and a pill when there doing the ultimate in hair loss actually cloning an abundance of hair . and isn’t junji Fukuda with his company trichoseeds also planning on starting human trials next year for hair cloning. Admin is there any new news from junji Fukuda?
This is essentially still cell injection I guess? Not what we usually expect as cloning. But if the area still got hair then it probably will still work pretty good.
Stem cell/iPSCs, 3D/4D bioprintin, gene editing therapies are in early development worldwide, but they will be very expensive. No doubt about it.
They have massive potentional in medicine, but we must build effective and cheap infrastracture to transfer in daily use. For example, one treatment with iPSCs now cost more than 400K (in preclinical trials).
It is very good sign that credible and rich pharma company from South Korea stand behind Epibiotech. Probably they see potentional in Epibiotech.
Why does the Epibiotech News page have only 18 views and nearly all views come from me ??
Are the people not interested in such a hair cure?
http://epibiotech.com/en/pr/?uid=70&mod=document
Funny. This is the full news page:
http://epibiotech.com/en/pr/
New very good news from Epibiotech:
Epibiotech (CEO Sung Jong-hyeok), a research and development company specializing in hair loss treatment, announced on the 14th that it has obtained a ‘human cell management business’ license in accordance with the High-tech Biopharmaceutical Safety and Support Act (hereinafter referred to as the “Anti-Biomedicine Act”).
https://www.yakup.com/news/index.html?mode=view&cat=12&nid=273306
J’en and how much ?… I could afford 100K max but if it is a one time thing… Otherwise maybe 5000 a year I guess max…
i need this now or in a couple of years, i am 40 already…not when when I am 55
Hi Admin , I thought of sharing this clip
https://youtu.be/Q65BI_5lul4, an interesting watch.
Article on Citrizine 1% topical
https://pubmed.ncbi.nlm.nih.gov/35976065/
Have you guys also already heard of “Mane Biotech” which started at Max Planck institute? I had registered for their newsletter months ago and I got a mail from them just a few days ago which said that their product was two times
faster and six times more effective than Finasteride + 100% of participants had stopped their hair loss and initiated regrowth. Also they wrote that they have a special launch in early 2023.
Unfortunately, I could not find much of information but do you guys know anything more of them? To me it sounds promising but so have already much more before…
I was in touch with them a few times, and the wanted to remain mostly in stealth mode till next year.
It should be a mechanical device, which people are wearing 30 min / day. Sounds like a massage device.
Source: https://www.deutsche-startups.de/2020/10/28/mane-biotech-haarausfall/
Two of the original founders already dropped out last year.
That’s a very bad sign. Why would they if this works? It would be a goldmine.
Folks, don’t get your hopes up. This shouts „scam“.
We as a community need to call out bs instead of adoring those „scientists turned entrepreneurs“ who play with our vulnerability to get our money.
Their web page seems to promise a miracle, but why are they so silent about it?
The hints of mane biotech “no drugs”, “no surgery”, “at-home-device” lead to a massage device.
A massage device is a way you can do something good for your hair. It’s proven that massages improve hair groth.
But if a massage device costs a lot of money, people will do massage on their own as alternative…. And why takes it 18 years of development …..So this kind of supporting hair growth needs to be cheap.
The only three jobs mane biotech offers in the career section are marketing jobs. That`s not a good sign. A really good treatment wouldn`t need much marketing. And the promises they do if it`s just a massage device are a little bit exaggerated…
Epibiotech sounds a little bit more scientific and mane biotech sounds more like a student project. Nevertheless they could still make money out of it.
It’s not a massage device, it’s a device/cap wigh a lot of tiny diodes which sends low-level electronic signals/pulses through your scalp, which in theory should stimulate the hair stemcells to start dividing again.
So no it’s not a massage device nor a laser, it’s a completely different mechanism. Make of it what you will, but it’s not fair to condemn it right away based on a false assumption.
I’m not sure myself what to think about it, we will only know once it’s on the market next year (if it actually does)
Thats also in regards to your point that they currently looking for hire in marketing, I mean yes, of course they would, they want to make a market launch next year after all.
Lorence they are silent for one of two reasons:
1) it’s bs and doesn’t work
2) it works but nothing great and they don’t want people to be disappointed too early before their trials are done.
My gut tells me this will work like a laser helmet. Maybe grow a few hairs here and there.
We shall see but I’m not expecting anything better than minox or fin tbh.
If they offer us another helmet to put on our heads we are in a bad spot
Sorry but it smacks of utter BS that mane biotech, low grade website, staff really nothing of consequence and ridiculous grandiose claims – 6x as good as fin lol come on
This is a bs as they come I’m afraid, if they had this the scientific community would be ablaze with it and tens of mills $ flooding in
A helmet! So laughable. I’m sorry, but I have ZERO faith in a helmet. None. And I doubt they’ll have any real evidence (that will hold up to scrutiny) of results from it. The days of helmets are long gone. People (most, anyway) are too smart to fall for that.
New interview with Stemson https://wxpress.wuxiapptec.com/geoff-hamilton-co-founder-ceo-stemson-therapeutics/ Geoff stated they have successfully completed their pre-clinical trials.
He worded it a little differently. You should re-read it.
Epibiotech has given precise milestones, while Stemson remains very vague. If there is not a clear statement on the following milestones soon, this company could lose confidence.
The news from their side is more about personnel decisions than actual results.
Im confused by this, would this work in areas without hair. Could you build a new hariline with this technology?
From my understanding you inject an area and hair starts growing? Or is it more like stemson?
Update via e-mail from a reader:
http://www.medifonews.com/news/article.html?no=172210
Update:
https://www.pharmnews.com/news/articleView.html?idxno=215348
“An official from Epibiotech said, “If the clinical trial plan submitted this time is approved by the Ministry of Food and Drug Safety, we plan to initiate phase 1 clinical trials in the first half of 2023.”
Another one:
https://m.mt.co.kr/renew/view.html?no=2022120916024380501
Another cooperation from Epibiotech. This time regarding their antibody-treatment.
http://epibiotech.com/pr/?uid=186&mod=document&pageid=1
Thanks Ben.
There is a new update in epibiotech.
Big promises – but the company seems solid and it would not surprise me if a cure came from South Korea in the future due to a very supportive government.
I hear it is allogenic….well if you have diffuse thinning, and you get cells from someone else…won’t it look weird ?
Why not taking your own resistant hair cells at the very back of your head if possible?
Bro any updates on junji Fukuda/trichoseeds starting human trails for hair cloning this year?
Please try to post in that subject’s related post.
https://www.pharmnews.com/news/articleView.html?idxno=218411
There is a recent update in epibiotech.
https://www.pharmnews.com/news/articleView.html?idxno=221863
They have 100s of news items like these.
Collaborations, cooperations, patents, approvals.
It’s all good and great, but after 8 years of operation I expect nothing but the start of a human trial. Everything else has become a nuisance.
Still believe they are 100 % legit though!
Another new and detailed interview with the CEO:
https://www.cosinkorea.com/mobile/article.html?no=48243
They definitely have the most approaches to hairloss treatments. But timelines are disappointing as always: 2026/27 is mentioned for their lead product EPI-001.
I don’t know if this particular interview has been posted on here before but the CEO states when he believes Epibiotech can become commercially available (2026-2027), believable considering Korean’s fast tracking of regenerative medicine. https://cosinkorea.com/mobile/article.html?no=48243
This treatment have to be repeat in every 3-5 months to have decent results.
In my opinion pointless…
How? The hair’s being picked out are from the donor region which are DHT resistant where did you get that number 3-5 months from? An interview, a science article?
Harvard Medical School posted news of successful regeneration of the hair cells in the ear as part of a research on hearing loss.
https://hms.harvard.edu/news/scientists-regenerate-hair-cells-enable-hearing
New interview with Epibiotech good to know that they still continue to plan phase 1 clinical trials of EPI-001 within this year.
https://kr.aving.net/news/articleView.html?idxno=1778926
https://www.youtube.com/watch?v=hKBkHGlZJf8&t=208s&ab_channel=AVINGNEWS_%EC%97%90%EC%9D%B4%EB%B9%99%EB%89%B4%EC%8A%A4
https://news.mt.co.kr/mtview.php?no=2023072516370239603 “EPI-001, a dermal papilla cell therapy, and is scheduled to enter phase 1 clinical trials within the year” Does anyone have any faith in them doing that this year lol?
https://www.chosun.com/special/special_section/2023/11/17/RSXTW2GQIVB5XMSOJN7OYCXQPE/
Thank YoYo! They gave the same dates previously, so I hope the 2026/2027 works out. I will be more confident once they get into Phase 2 trials.
No problemo, I’m just happy that they finally addressed how often the treatment would be needed. Even though once a year is better than a few months it still isn’t ideal :/. However, I’m glad that they are keeping to their word that clinical studies should be starting before the year’s end.
Green light from the authorities in South Korea.
https://news.mt.co.kr/mtview.php?no=2023122109175632291
So in 2024 there’s 3 companies that are officially entering cell therapies in human trials. As far as I understand Epi‘s trial is planned to be finished in the 2nd half 2025 which makes a commercialization in 2026 possible?
Nice find Ben! I didn’t actually believe the CEO when he said they’ll get the go by the end of this year
Thanks Ben. So much good news from South Korea. 2026 definitely seems possible for autologous, though I am not sure about their national regulations.
Crazy where the country’s population is headed though:
https://time.com/6488894/south-korea-low-fertility-rate-trend-decline/
I’m genuinely not trying to be a doomer etc., but I’m confused as this exact hair therapy is exactly what Replicel and others have been touting and there’s never any result?
Replicel/Shiseido is dermal sheath cup cells.
Epibiotech is dermal papilla cells. A few other companies are doing similar work (see second and third paragraphs of today’s update).
Do they plan on starting hair follicle cloning trials any time soon?
I don’t quite understand the term “phase 1/2 A clinical trial”. The truth is that I have never been very familiar with Epibiotech, but if its EPI-001 treatment consists of the injection of autologous cells from the dermal papilla, I do not understand the sense of naming this technique as if it were a medicine, nor do I understand the sense of naming phases within a clinical trial, when the only possible phases for this method is to carry it out and check if it is effective, since this method is autologous and in theory there should be no more complications…
Epibiotech recently uploaded english presentation on EPI001. I hope they soon start their long pending clinical trial.
https://www.youtube.com/watch?v=LY4Ln-qjFN4