Today’s “The Telegraph” newspaper from the UK has an interesting article titled:
“Are we witnessing a new dawn for baldness treatments?”
Unfortunately, the article is behind a Paywall, but I have highlighted the main points further below.
Interesting New Baldness Treatments
As is the case with all such clickbait titled articles, a lot of the information is not new to us or is exaggerated. Nevertheless, I do believe that the past 5-10 year period has been the most groundbreaking in hair loss research history. And this article has some interested new information.
Dr. Coen Gho
Amazingly, the first ten paragraphs of the article are devoted to Dr. Coen Gho and his Hair Science Institute! For anyone who has been reading about hair loss treatments for more than 15 years, Dr. Gho is a legend as well as highly controversial. Please read my past post on Dr. Coen Gho and his hair multiplication technique.
Dr. Gho’s method attempts to avoid donor area hair thinning by only using part of the follicle during a hair transplantation procedure. It supposedly works by stimulating the stem cells within that small piece of tissue to generate new hairs. At the moment, this supposedly yields two hairs from a single follicle fragment. However, Dr. Gho is now developing a separate technique that could potentially generate 10 hairs:
“Gho has just gained approval to test the technique in female patients in research studies. If all goes well, he hopes that it may be possible to offer it as a treatment in the next four to five years.
Right now a typical treatment involves taking grafts from 1,400 hair follicles, which means 3,000 new hairs,” says Gho. “Can you imagine what we can achieve if we could use those same grafts to generate 10,000 hairs?”
Please note that there is significant debate and controversy about Dr. Gho’s technique and its efficacy.
According to Dr. Gho, current hair transplant demand at his clinic is 3-4 times higher than before the Covid pandemic. This makes sense per various hair transplant statistics. All my hair transplant advertisers also backed out this year since their clinics are too busy through Spring 2022. They do not want to keep rejecting new patients! Zoom, Teams, FaceTime, Instagram and more have really pushed people into getting cosmetic surgery at a record rate.
Cassiopea and Riken
In this article, Cassiopea CEO Dr. Diana Harbort is quoted as saying that there is a major need for a novel hair loss treatments. She gets weekly e-mails from people trying to enroll in trials for the company’s hair loss product Breezula.
Riken and Dr. Tsuji are discussed in great detail, especially his attempts at crowdfunding (they only need $3.2 million). According to Dr. Tsuji:
“As soon as we can get the funding, a clinical study could be started within a few months, and a pay-to-participate clinical program could begin within two years in Japan.”
Dr. Ke Cheng and Exosomes for Baldness
Of most immediate interest to me is a section on exosomes as being one of the new array of baldness treatments. It is preceded by a discussion of PRP for hair loss.
I have covered exosomes in detail in three past posts. There was also a presentation on this treatment at last month’s ISHRS conference (where it was discussed favorably, albeit with warnings about new FDA regulations).
In this latest Telegraph article, the author interviewed Dr. Ke Cheng from the Cheng Lab at North Carolina State University. Mr. Cheng is a professor of regenerative medicine and his research interests include exosomes and micro-RNAs. His team published a paper on exosomes and hair growth in 2020:
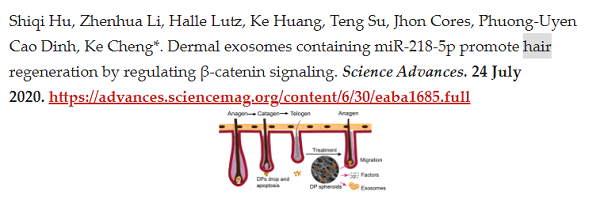
Dr. Cheng’s Lab is using exosomes from healthy hair follicle cells (which also contain microRNA) and injecting them into balding regions of the scalp. These “fresh” exosomes send messages to the hibernating cells to promote hair regrowth.
“So far Cheng has tested this approach in mice and found that it can achieve a six to seven-fold increase in hair growth compared with traditional hair loss drugs such as minoxidil. He is now conducting experiments to see whether the same results can be achieved in human hair cells in the lab.”
Dr. Cheng plans to start clinical trials in “the next five years” and is actively speaking to venture capital firms. Kind of a strange time frame, since so many hair transplant surgeons already offer this treatment.
Even with strict new FDA regulations, it seems like some doctors are still treating patients with exosomes. Note that these extracellular vesicles are derived from another person, so are not classified as autologous in nature.