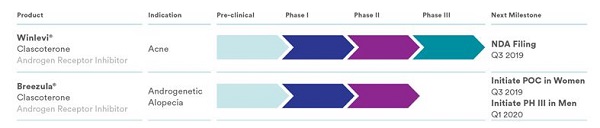
Breezula® (clascoterone solution) is a novel androgen receptor antagonist that is currently in Phase 3 trials for the treatment of androgenetic alopecia (AGA). If approved, it will be sold by Cosmo Pharmaceuticals NV (Ireland). Breezula was originally called CB-03-01 and manufactured by Cassiopea (Italy), prior to the latter’s 2021 takeover by Cosmo. Breezula product details can be seen here.
I have written about Cassiopea/Cosmo/Breezula in well over a dozen past posts. Feel free to read all of them, going back to the very first one in 2014 when the product was known as CB-03-01. Its key ingredient is known as clascoterone, an androgen receptor (AR) antagonist. Similar to Kintor Pharma’s KX-826 (Pyrilutamide).

Update: March 21, 2024
Breezula will come out in 2026 and cost $110 per month
Cosmo Pharma just released a detailed new pdf regarding the company’s 2023 full year performance. Breezula details are from page 31-39. Some great new information that suggests the company is very confident about Breezula (clascoterone) being approved and coming to market at the end of 2026 in the US and EU.
The US launch price will be $110 per month. Cosmo plans to target the existing physician-driven AGA market where doctors usually prescribe their balding patients oral finasteride or OTC topical minoxidil. Breezula expects to capture 35 percent of this market.
Update: March 20, 2024
Breezula Phase 3 Trials Update
Cosmo Pharma just reported excellent full-year 2023 financial results and doubled its dividend payment. More importantly, regarding the Breezula (clascoterone solution) Phase III trial in males for the treatment of androgenetic alopecia:
- 348 out of a planned 726 patients have been recruited in study CB-03-01/37.
- 507 out of a planned 726 patients have been recruited in study CB-03-01/38.
The Phase 3 trials are across four countries (US, Poland, Germany and Georgia) and 50 centers.
Update: September 13, 2023
Breezula Phase 3 Trial Enrollment Opportunities
Edit: It seems like there are around 30 US locations. You have to scroll through a separate box in the SCALP1 and SCALP2 links above.
================================================
Earlier this week, someone I trust sent me a link in regards to a new androgenetic alopecia related clinical trial that is taking place in New Jersey, US. There are two very interesting keywords in the URL that I was sent:
https://cenexelresearch.com/hri/trials/androgenetic-alopecia/?utm_source=Facebook&utm_medium=Paid&utm_campaign=Cassiopea%20CB-03-01%2F37&utm_content=Dermatology
The shortened version of the link on CenExel clinical research organization (CRO)’s androgenetic alopecia page does not mention the involvement of Cassiopea (or its new owner Cosmo Pharmaceuticals). Neither does it mention any product name.
However, in my last update of this post in June, I mentioned that Cosmo is finally starting its Breezula topical anti-androgen Phase 3 clinical trials in the US and Georgia. A total of 750 males will be enrolled between the two countries. The trials will end in January 2025. The above mentioned trials in New Jersey are almost certainly for Breezula.
Note that you have to be a male of 18 years age or older who has androgenetic alopecia.
Update: June 29, 2023
Cosmo Begins Phase III Trials for Clascoterone (Breezula)
Cosmo Pharmaceuticals just announced the beginning of Phase III trials of clascoterone solution (5%) in males for the treatment of androgenetic alopecia (AGA). The name of this product is Breezula® and it is a novel androgen receptor inhibitor. Trials will involve 750 patients in the US and Georgia. The company’s acne product with the same clascoterone ingredient (but lower dose of 1%) is called Winlevi and is already available in the market.
Key quote:
“If approved by the FDA, clascoterone solution has the potential to be the only topical androgen receptor inhibitor for AGA and the first drug with a new mechanism of action for the treatment of AGA in nearly three decades.”
Update: February 16, 2023
Cosmo Pharma’s latest guidance states the following:
“Cosmo expects to have the first patient enrolled in the Breezula® (Clascoterone solution for androgenetic alopecia) phase III trial in males in Q1 2023.”
Also see Cosmo Pharma’s new page on Breezula. The company took over majority ownership of Cassiopea in December 2021 after being a part-owner for years (see bottom of this post).
Update: October 7, 2020
Cassiopea (Cosmo Pharma) completed enrollment in its Phase II trials for female pattern hair loss. The 6-month study has enrolled 293 patients. The four-arm study will divide volunteers into four treatment groups: clascoterone solution 5% BID (twice daily); clascoterone solution 7.5% BID (twice daily); minoxidil solution 2% BID (twice daily); and vehicle BID (twice daily).
Update: August 27, 2020
Cassiopea’s Winlevi topical androgen receptor inhibitor has just been approved as an acne treatment by the US FDA. It is the first acne treatment with a new mechanism of action to be approved in 40 years. The key ingredient (clascoterone cream 1%) is the same as in the keenly awaited hair loss product Breezula. The latter will have a higher dosage.
CEO Diana Harbort quote:
Dermatologists have said targeting androgen hormonal activity in the skin is ‘the holy grail’ of acne treatment for both males and females.
I expect Breezula will not come to the market till 2023, assuming it gets final approval. However, some people plan to use Winlevi on the scalp, even though this is not recommended by the manufacturer.
Update: March 19, 2020
Phase 3 Trials Delay
How things can change in just a few weeks. Breezula Phase 3 trials have now been delayed till 4th quarter 2020 or 1st quarter 2021. All due to COVID-19 (Coronavirus), which has hit Italy particularly hard. More information can be found in Cassiopea’s 2019 Annual Report which was released today.
The Clascoterone female pattern hair loss Phase 2 trials are on track to finish enrollment in 2Q 2020. Trials should be completed by year-end 2020. However, delays are still possible depending on progression and recurrence of the current pandemic.
Quote from the accompanying pdf:
“As part of the process in the NDA review, the FDA amongst other, also inspects the facility in which Clascoterone cream 1% is manufactured. Clascoterone cream 1% is planned to be manufactured at Cosmo Pharmaceuticals plant in Lainate in Italy. Given restrictions currently in place because of the COVID-19, the FDA had to postpone the inspection planned for March.”
Nevertheless, Cassiopea is still planning to release its Winlevi acne cream product by the end of this year.
Update November 13, 2019: Cassiopea’s women’s hair loss clascoterone solution Phase II trials starting soon in Germany.
April 16, 2019
Cassiopea (Cosmo Pharmaceuticals)
I have covered Italian company Cassiopea since 2014 (when it was called Cosmo Pharmaceuticals). The company is developing a topical anti-androgen hair loss product named Breezula (originally called CB-03-01). The chemical name for this product is Clascoterone. Note that Cosmo Pharma still owns 45 percent of Cassiopea.
Breezula will be available for both men and women. Several of my well known physician contacts in the hair loss world have told me great things about this product. Their feedback is based on what they saw at conferences or heard from colleagues.
Breezula Phase II Trials Very Successful
In July of last year, Cassiopea stated that the interim Phase II clinical trial results for Breezula were very positive. Today, they released twelve month Phase II trial results with the same conclusion:
Cassiopea announces very positive Phase II Twelve Months Results for Breezula® (Clascoterone) in treating androgenetic alopecia.
The presentation of the results is confusing, detailed, full of acronyms and somewhat open to interpretation. Note that “BID” in there stands for “twice per day” after conversion from Latin. Hopefully they release before and after photos at some point.
Also note that the previous 6-month interim report had 375 subjects, and the 12-month report has 344 subjects. The trial was conducted in Germany.
Breezula Target Area Hair Count Increase

Best case result highlighted in yellow (14.3 new hairs per square centimeter, after 12 months of 7.5% BID clascoterone solution dosage). BID means twice per day.
Breezula Target Area Hair Width Increase

Best case result highlighted in yellow (762.5 um width increase, after 12 months of 7.5% BID dosage).
My gut feeling is that Breezula will be at least as effective as Finasteride and Minoxidil.
Clascoterone Summary
Some key points from the above linked article from Cassiopea:
- If approved, Breezula (Clascoterone) will be the first FDA-approved topical anti-androgen for the treatment of androgenetic alopecia.
- Moreover, it would also be the first new drug approved for treating androgenetic alopecia since 1997. That year was when Propecia (aka Finasteride) was first approved to treat hair loss. That year was also when Tiger Woods first won the Master’s golf tournament. His 5th surprise victory in that tournament came this past weekend.
- Based on these great results, Cassiopea plans to proceed with 6-month Phase III trials in men in the fourth quarter of 2019, after consultation with the FDA. The company is also going to start proof-of-concept trials in women.
- Breezula works by blocking dihydrotestosterone (DHT) interaction with hair follicle androgen receptors.
- Clascoterone does not interfere with testosterone and other hormones in male subjects. Libido and sexual behavior changes have so far not been observed in clinical trials. Moreover, due to its rapid metabolism and localized activity, Clascoterone (Breezula) does not produce any systemic side effects.
In the past, I used to often state that perhaps an initial cure for hair loss would entail the usage of a cocktail of products. Each working via different mechanisms and chemical or biological reactions.
It is looking likely that Breezula will be one of the main products in this cocktail.
Note that Clascoterone 1% cream to treat acne will be released by Cassiopea before Breezula. The acne product is called Winlevi.