I covered Follicum (Sweden) and its FOL-005 (now FOL005) hair growth peptide in eight posts between 2015 and 2021. Prior to the company’s cessation of work on FOL005 and a 2022 acquisition by Coegin Pharma (Sweden).
Follicum’s unique osteopontin based hair loss treatment is very interesting. Their product can both stimulate scalp hair growth and reduce excessive body hair growth (hirsutism). And the same technology could potentially treat diabetes and inflammation related problems.
Follicum’s frequent press releases, CEO Jan Alenfall’s presentations, and the company’s rapid clinical trial progression impressed me. Their chief business officer Gunnar Gårdemyr was also in regular communication with me.
October 25, 2023
Coegin to Release FOL005 Hair Growth Peptide Gel in 2025
Some major new updates since I wrote the summary earlier this year (see second half of this post).
- October 17, 2023: Jens Eriksson becomes acting CEO of Coegin Pharma.
- August 31, 2023: Coegin shares a video update on its FOL005 development strategy.
- August 24, 2023: In its interim report, Coegin states the following (also see another summary here):
“After carefully analyzing the possibility of launching FOL005 as a cosmetic product line, we have come to the conclusion that it is an opportunity with great potential. This will minimize development risk, significantly reduce capital requirements and strengthen commercial opportunities in the short and long term. We have therefore revised our strategy and now aim to launch FOL005 as a cosmetic product series as early as 2025 with the USA as the first market.”
It seems like CosmeRNA started a great new trend. No more ten year clinical trials and failure to come to market!
- Follicum’s website seems to have been restored and updated very recently. The milestone plan page has a detailed chart for FOL005:
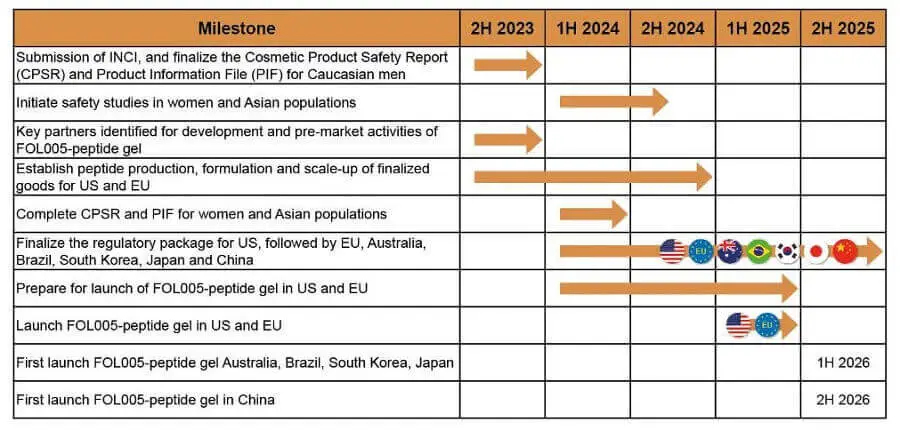
April 8, 2023
Follicum Past Clinical Trials
I covered both of Follicum’s past clinical trial results in 2017 and 2021, respectively.
- In 2017, Follicum’s Phase 1 clinical trial results were released. They were deemed as positive (8 percent increase in hair growth), with an effect comparable to that of Minoxidil. Moreover, safety was not an issue, with no major side effects in trial participants.
- In 2021, Follicum’s Phase 2 clinical trials of FOL-005 came out. The topical compound resulted in a 6.6 hair/cm2 increase in hair growth, but this was deemed to be insignificant compared to placebo.
Thereafter, the company ceased further development of FOL-005, and it all seemed over for yet another false flag operation. Or perhaps not?
Merger and Acqusition by Coegin Pharma
In September 2021, Coegin Pharma merged with Follicum. This merger was confirmed as an acquisition in 2022.
In the first of the two links above, Coegin’s CEO Tore Duvold said something of interest in the interview:
Question: “With regard to the hair loss project, which has recently been put on hold, how will it be handled once the companies merge”?
Answer: “Follicum announced that they have paused the hair loss project. In Coegin, we have strong capabilities in dermatology, and we will review and analyze the results before we make any conclusions”.
Follicum Back in the News
- In March, a Tweet from Coegin indicated that they were meeting with potential partners for Follicum’s FOL-005.
- Follicum was mentioned in February in an updated article titled: “Could We See a New Dawn for Hair Loss Treatments?”. It summarizes the problems that the company faced in trying to bring FOL-005 to market after the unimpressive Phase 2 trial results.
- When going through Coegin Pharma’s website, I found a highly detailed comprehensive corporate presentation on Follicum and FOL-005. It was presented at the end of 2022 by Coegin’s COO Kristian Lykke Fick.
Their take on the past trials is more positive. Key quote:
“FOL005 1.5% dose was on par with treatment effect reported for minoxidil and finasteride with a growth of 12 hairs/cm2 after 4 months of treatment. However, with more than 60% of subjects responding to treatment compared to competitors where 40% responders effect is previously documented after 4 months.”
Note that Follicum’s old Swedish website is being routed to the English site that has an expanded research section on FOL-005. In 2022, they also filed for a patent related to wound healing. I am surprised they do not mention anything in relation to their osteopontin-derived peptide’s body hair growth inhibition.
Follicum plans to conduct various new scalp hair growth related clinical trials from 2023-2027. On their website’s plans section, they state the following:
“We believe there is a high likelihood of success based on the previous clinical data.”
Too bad about the new time frame and the wait for venture partners for funding. Otherwise, I am not entirely pessimistic about this product. I do feel that it can compete with Finasteride and Minoxidil in terms of effectiveness, but via a totally different mechanism of action. And with no serious side effects.
I am underwhelmed.
Yea Follicum needs to quit while it’s still behind.
I’m really tired of this kind of news. experiment experiment experiment. they are really making fun of us. Do we look that stupid? I ask those who hear my voice; Please let’s protest these fake news, experiments, companies. That ‘s enough.
I like your idea. How do you think we should go about this protesting? I think our angry comments are making a statement. It would really be great if these companies actually read our comments. So many companies working on hair loss and it seems only the products that don’t work get to market but anything that could actually work to some degree is delayed to infinity. It’s quite a disappointment.
Nobody is impressed with something comparable to Fin/Minoxidil. At this point, they’re just wasting investor dollars.
If Histogen also comes back, I will be scared to write a post in case all regular readers leave for good:-)
Admin, I’m also afraid you will update the name of the site to Balding By 2030 :)
I’d be first in line if this, Histogen, Follica or any other abandoned/laggard actually came to market. As far as the timeline for FOL-005 I’m not shocked, it’s basically the perpetual hamster wheel of 5 years running!
Really hoping CosmeRNA works well and comes out soon to purchase. I want to add it to my regimen.
These companies are a pathetic joke. No one with hair loss needs another Finasteride or Minoxidil. It’s getting so old. Pyrilutimide is another one that is just a lot of hype. I’m using it in my recessed temples and seeing nothing in terms of regrowth. $110 down the toilet. I’m tired of these companies and their garbage products which all don’t work! What we need is hair follicle neogenesis related companies. Once the hairs are dying off, saving them is difficult if not impossible most of the time.
I’m waiting for these folks at Stanford University to begin human trials with Verteporfin and FAK inhibitor VS-6062. They seem to show actual results and I have some confidence they may even work with microwounding as opposed to deep wounding. However it’s been 2 years and they’re doing nothing regarding human trials to this point. Give the Longaker Lab there a call and tell them to get off their behinds and start clinical testing already!!
The whole industry is all about money, selling products to desperate people that don’t work, gatekeepers and institutions wanting their exorbitant pay for anything to be allowed to happen at all. It’s all a money grab! Pathetic. We’d have a cure by now if the industry wasn’t so lukewarm, unmotivated and corrupt. Stemson is a perfect example of this. They report nothing, hire people who accomplish who knows what and feel is not really worth us knowing. Dr. Tsuji has a hair multiplication cure that will be available when after the end of the world occurs the way things look. He says we’ll have a cure in 2026, more likely 2062. Clinical testing yet? Nope! G-d help us! With these pieces of work running things we’ll be bald to our graves.
@J.M.M. Please don’t knock Dr. Tsuji. He’s been wasting our time for years now and I need something to keep laughing at.
Don’t forget Replicel, that other heavyweight- Phase 2 soon!!
I heard from Andrew Schutte of Replicel they’re beginning their treatment for hair loss in the Caribbean now. Wonder how that’s working out. I see their stock is moving up slowly. A good sign maybe? Need to look into what’s going on. I think it’s good for androgenic alopecia only though.
https://www.nature.com/articles/s41598-022-10024-2
Corrections to the CosmeRNA results in the studiea report were issued 5th April.
Says 5th April 2022.
I checked their website and apparently they intend to release their product in the first half of 2025 in USA/Europe. I am perplexed because : 1) no phase 3 trials ? 2) 2 years ago they said only 6.6% more hairs in their phase 2a, but now on their website it magically went up to 12%.
I was thinking of writing one my next posts on Coegin. The last article I read said they will release it as a cosmeceutical in 2025.
Coegin Pharma mid-year 2023 full report:
https://cdn.bequoted.com/media/1/5bc1f84d-8750-4328-8e2e-f3fc35d703de/Delarsrapport-Q2-2023.pdf
Of course, cosmeceuticals mean quicker availability, but also less evidence of effectiveness. It’s interesting though, as Amplifica’s AMP-303 appears osteopontin-based and, according to their CMO (youtu.be/2-dyC4F0AvM?si=MQKr_W4WLq65w7wv), will only need limited clinical testing (their recently initiated trial title sounds Phase 3).
Thanks LJ for this underrated comment including the link.
@admin: I think this information is absolutely precious. I just went through the video (horrible quality) and Dr. Rassman said that 2 of the 3 candidates (probably 203 and 303) are already in use as medicines for other indications than hairloss, meaning safety is already established.
Now I see why they stalled SCUBE3 – of course you start with the „fastest to market“.
I hope this becomes the homerun we are all waiting for. Strangely enough everybody (including me) automatically assumes a quicker route or a cosmeceutical is less effective or even a scam. Dr. Paus also believes his tastants/odorants are as good as fin/minox.
Thanks Ben, I also mentioned in another comment section about the replicel injector, as it seems AMP-303 would initially have to be injected (to precise depth) before eventually being developed into a topical.
Still if AMP-303 came to market with FDA approval in the next couple of years, why would anyone buy FOL005?
It doesn’t matter eventually. If a product comes to market, it will be bought. If it fails as an effective treatment it will vanish again, until then millions will be made. And that’s independent of better products available.
Replicel‘s injector was made with the intention of injecting living cells, which probably needs different properties than medical molecules. Maybe they changed the tech later, I don’t know.
Besides that, there’s plenty of injectors on the market. That’s nothing novel. Just have a close look around in a dermatologist‘s office.
Also: Stemson, Fukuda, and Tsuji have their own devices, protected by patents. But that’s transplants – different again.
Makes sense, though Rivertown had a product with 2-3 already approved ingredients if I recall. Yet the company folded.
Do you know what stage of trial they reached? Amplifica may fail yet if they can’t demonstrate the clinical efficacy for FDA approval…
Maybe fol005 is competing with amp303 and they know that Amplifica will be successful and want to bring their product with less effectiveness onto the market as a cosmetic product beforehand in order to benefit from financial success. And later when amp303 is on the market they will disappear because they can not compete with they effectiveness!
I wonder about this as well. They (Plikus/Amplifica) must believe their product is unique and likely to be more effective than FOL005. I’d love to be a fly on the wall listening to what Plikus/Amplifica make of FOL005, and how their product is *actually* different. I could see Coegin wanting to capitalize, but I also see where AMP-303 will potentially run into similar problems if they have solved the delivery method/vehicle issue that a molecule this size is.
Yeah comseceutical prolly because it works as good as samrna.
If they pretend to treat diabetes as well I guess it should be more than cosmetical ?
How professional folliclepharma states that kx-826 is taken orally. Great company go for the cosmetical route. I always recommend cosmetical route for products that don’t even work.
“It seems like CosmeRNA started a great new trend”. This is bad imo because those are unproven treatments. If it is as good as finasteride/minoxydil, surely they can find investors to make a legit phase 3 trials. Sounds fishy.
I have yet to see any hair loss company (finasteride and minoxidil already existed as medical treatments) bring any product to market. Even after great Phase 2 trial results, and 10 years of delays before folding. This shortcut is needed for now I think. I would rather waste a few hundred bucks on a cosmetic that turns out to be minimally effective versus waiting for 10 years again.
Kintor is an outlier when it comes to its speed of progress. They have zero issues with funding.
Hello admin, that is my thinking as well. BUT, I would never stop using proven and effective treatments. There may be synergistic benefits we don’t know yet.
Luckily I don’t have to worry about cost. But I hate using topicals daily. Still have nightmares using liquid minox decades ago…..
Well, another doubtful potential treatment…
However what makes it different is that they talk about diabetes, that is interesting somehow and it means it’s not solely a treatment that works on androgens…
Again I know I already commented with those links, but am I the only one who think it is interesting ?
Anybody has read this about metformin :
Metformin Promotes the Hair-Inductive Activity of Three-Dimensional Aggregates of Epidermal and Dermal Cells Self-Assembled in vitro
https://pubmed.ncbi.nlm.nih.gov/34883492/
https://www.cfspharmacy.pharmacy/blog/post/topical-metformin-for-alopecia
I am surprised I am not reading more about it
Have to agree with the prior posters on comseceutical route Admin. A quick buzz to see something come to market, then the crash when reality sets in that it most probably doesn’t do anything except lighten your wallet. I prefer to wait for something effective having been taken advantage of by many a snake oil salesmen over the years. They are getting much more adept at making their products look legit e.g. Brotzu.
Yes but they talk about it affecting diabetes ? Or they’re just lying to get a buzz… You can seel something and say it can improve diabetes if it is only a cosmetic ?
I would be surprised as well if it worked anyway..it seems those company are maybe making more money creating a buzz than actually searching for something that works…
The topical metformin however seems really interesting to me as it has been proven to regrowth hair on women with cicatricial alopecia !
Hello All,
I hate to be a Debbie downer, but is there ANY evidence at all to suggest that a cure is even possible, let alone imminent in our lifetime? What if there is no cure, and once the follicles are gone, they’re gone forever? Just as we cannot reverse death, what if the regeneration of a hair follicle is just as daunting/impossible? No one wants a cure for this dreaded affliction as much as I do, but I just cannot see anything on the horizon coming to rescue our tortured souls. I wish people were working to change this, but I am not confident.
If the recent pig heart transplant lasts, we could one day soon transplant animal hair to humans.
I see Fukuda as the closest to a cure. Also Tsuji. Stemson a little later to the party. All three are complete cures potentially. Epibiotech may be up there too.
Then Technoderma, HopeMed, Kintor‘s
PROTAC. Amplifica as a wild card. Pelage will be a strong contender.
I am as optimistic as never before, bear in mind: we‘re closer than ever. Yet years away, that’s clear!
@admin: are you following Fukuda‘s presentation tomorrow?
Can you remind me where watch Ben!?
Sorry I can‘t find it…in some of the press releases there was a statement that the whole presentation can be watched online for free after registration.
There’s also no information on Fukuda‘s website. Apologies.
Don’t count out replicel and hairclone! I also think niostem may be a wildcard.
I am sorry to disappoint you.
All three you mentioned are complete failures. Hairclone and Replicel exist since the early 00s and haven’t been successful at all.
Niostem is close to a scam.
OK, your guess is as good as mine really. Time will tell…
Except my friend, it’s not a guess at all.
Replicel did trials, and Niostem is on the market for almost a year now.
All authentic results of Niostem were not satisfying, and underwhelming at best. The marketed pictures were the best responders and possibly skewed because of additional intervention methods and/or photo editing.
Shiseido/Replicel published the outcome of their trial, it was simply disappointing. Since then there was no follow-up. DSC-cells are definitely not the way to go.
Hairclone failed already 15 years ago under the name Intercytex. Paul Kemp is announcing the launch of his product since 2016 for every year for the following year. And he does that until today. They are also notoriously underfunded. But you need millions above millions to pull this venture through. I guess his approach is scientifically sound though – but this will never come to fruition for various reasons.
Sorry Ben, but you’re low information. Niostem hasn’t even shipped yet and they’re in the process of setting up a trial with the first wave of users.
Replicel has all sorts of optimisation it plans to do wrt depth, dose and frequency. Who really knows what they’ve got under wraps with this arbitration ongoing?
Not as up on HC, but aren’t they starting trials in the UK next year?
Dude, what are you even talking about?
https://niostem.com/
Available for everybody since May. Limited releases were even done before that. I know a guy who is using it now for 6 months (with – surprise – no effects!).
Replicel had an unsuccessful trial – that’s all what we know and they have been quiet for years now. Everything else is pure speculation.
Hairclone is announcing trials for many years now always for the next year.
I bet none of the three will ever play an important role.
Either you’re lying, or the guy you know who claims to have it, is lying.
The test group only got it this week, and it’s not being released till January.
Dear Falcon, I am from Austria and Niostem is a German company and their product launch therefore was naturally in Germany/EU. I’ve been in contact with their support team several times in spring this year but decided to not try it (back then there was an extremely low price – it’s almost double now).
If you are outside of the EU you might not have had access to buying the device, I don’t know. But it’s been definitely available in certain countries for quite some time now
I also heard of delivery problems, apparently due to high demand.
Eventually it doesn’t matter – it’s not good enough to make a difference.
Company has confirmed Ben is full…
Anyone else can message them and they’ll say the same.
Nobody has had the device for 6 weeks, let alone 6 months.
So you can disregard his doomer comments.
Even not knowing that the delays are not rumours and something they outright stated due to having to change suppliers.
Your “friend” is lying to you. And you’re really missinformed on the entire thing, so it would be better to just stick to being a doomer and not spread lies.
Even just the thought that you’re friend could miraculously get the device months before the email looking for beta testers we’re asked to send in applicatios is wild.
I have been lettting all these comments through (barring one single sentence insult), but personally, I would not get too excited about any sound, electricity or laser based device. It seems that some of you are basing a lot of hope on Niostem being a cure. I am much more interested in Pyrilutamide, HMI-115, Breezula etc. and even those might not turn out to be anything close to cures. Though the HMI-115 macaque before and after image is exciting.
Dear Falcon, at this point I have to assume you are affiliated with Niostem,
based on your past posting history (as far as I can see you only comment on Niostem) and the way how heavily you defend that company including insulting people with different opinions.
Just one last remark to defend my claims regarding the device is not available: there were numerous experience reports on German alopecia forums from people who used the device. None of them were positive. Some were removed later (I can imagine there was pressure from Niostem).
Also a big point of criticism was the business model of Niostem: crowdfunding (why for this supposed superior product?), questionable claims (6 x Fin? lol) and intransparent or totally skewed results.
@admin: thanks for your voice of reason. I agree that Niostem won‘t be the solution we are hoping for. Also I see it as my duty as a veteran in this forum to warn the young sufferers from unreasonable high expectations of new products – some of them complete scams. I was a victim of fraudulent products too and it was not a good experience – healthwise and moneywise.
Cant reply, to that actually comment. But Ben’s just spreading doomer misinformation.
1. I’m from the EU, I know not available.
2. I just emailed them and nobody has had the device for 6 months, design mocks where about. So either you’re friends been sticking a mock on his head every day, or yous are just spreading misinformation.
Except for a few beta testers the people in the clinical trial are the only ones with the device, and they only got it between 4 & 6 weeks ago.
Dear Falcon – as said I cannot say how many people have actually used it I don’t have that kind of numbers. There‘s been rumors of heavily delayed deliveries and problems with the quality of the devices.
If you say it’s not available that’s simply not true and then you are the one spreading misinformation, not me. It’s been available for quite some time (call it „Beta-testers“ or within trials or whatever). A friend of mine uses the thing now for months, as said without visible results. I had the chance to buy it in spring – I think it was a limited release – but decided against it.
You can discredit me however you like, that’s okay.
Eventually there will be no significant results from Niostem.
Can’t reply to admin’s last comment. My apologies. However, isn’t niostem a device using electrical currents?
The only reason I think it’s interesting is that they tout results of ~40 hairs/cm2 without side effects, which, if proven, blows all competition out of the water.
You are correct. I said sound, laser and forgot the third category. Now added.
I will trust their claims more after further larger trials.
Any guess what happened toTsuji?
Last news was that he was waiting for funding and changed the company?
Maybe there was some level of success with HMI-115: https://www.biospace.com/article/releases/chime-biologics-and-hope-medicine-enter-manufacturing-agreement-to-speed-up-the-launch-of-first-in-class-antibody-drug-hmi-115-targeting-endometriosis-and-androgenic-alopecia/
oh wow – exciting!
This is good news. I’m a skeptic at heart but always had a good feeling about this one. Now I want to see photos from the last trial.