Thanks to readers “Ben” and “Theo” for the regular updates on Epibiotech (South Korea). Note that Stemore was renamed to Epibiotech in 2021. Since then, the new company has been in the news almost every month. Note that besides EPI-001, Epibiotech has several other hair loss products in its pipeline that I have listed at the bottom of this post. The top half is all recent updates.
Update: May 9, 2024
Epibiotech Receives Funding to Begin Clinical Trials
Epibiotech just received approval for government funding for its pending Phase 1/2a clinical trials. I expect these trials to begin before the end of 2024.
Per CEO Seong Jong-hyuk, this is the first clinical trial in the world to conduct an autologous cell therapy for hair loss using hair papilla cells. I am not sure if this is true, since Intercytex completed Phase 1 trials on its dermal papilla expansion and transplantation (vie injection) process several decades ago.
Moreover, the Fukuda Lab team in Japan recently announced that dermal papilla cell transplantation is about to begin in Japan. And perhaps Han Bio and HairClone have both further advanced their dermal papilla cell expansion related technology as of 2024.
New Epibiotech Video:
Update: April 23, 2023
Epibiotech has succeeded in growing hair in pigs via the injection of human dermal papilla cells (hDPCs). I discuss the details of this significant development in my post on pigs and hair growth.
Update: February 9, 2023
New Epibiotech CEO Interview
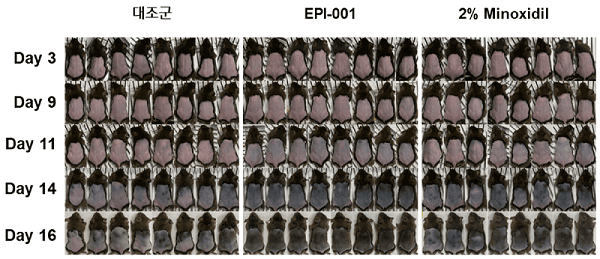
A very detailed new interview with Epibiotech CEO Sung Jong-hyeok. The translated title states that a powerful new drug for the fundamental treatment of hair loss is coming. However, Phase 2 trials are yet to begin for their main EPI-001 product. Key quote and photo below. Note that they are working on several different ways to tackle hair loss.
“There are many companies worldwide that conduct research on hair loss. But we are confident that Epibiotech is the only company that is developing various modality treatments based on cells, antibodies, and low-molecular compounds (chemicals) and that is conducting the most in-depth research.”
“In addition to the currently leading dermal papilla cell-based new hair loss drug, it is discovering and developing new hair loss treatment targets such as CXCL12 neutralizing antibody and RIPK1 inhibitor.”
Update: January 20, 2023
Epibiotech and Novelty Nobility sign an agreement to develop an antibody treatment for hair loss. Epibiotech plans to add any new antibodies discovered by Novelty Nobility to its own hair loss treatment protocol.
Update: November 22, 2022
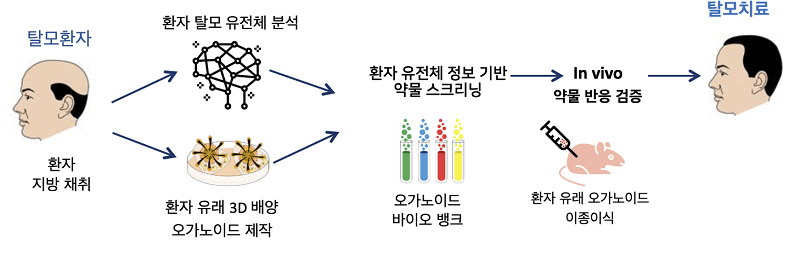
A new paper from the CEO on cell therapy for hair regeneration that necessitated my writing an entire blog post.
Update: September 9, 2022
Epibiotech and Humedix to Jointly Develop Hair Loss Treatment
Thanks to reader “Theo” for the below two recent updates. On September 7, Epibiotech and Humedix announced a new partnership. The two companies will collaborate to further develop Epibiotech’s hair loss treatment. Humedix seems to be a reputable cosmetics sector player, with significant experience in manufacturing injection and platform technology.
Moreover, according to the press release, Humedix is also developing:
“Spheroid culture technology through 3D culture of dermal papilla cells and a scaffold using biopolymer materials such as bio-ink related. The goal is to expand the business into the cell therapy field by combining 3D bioprinting technology and biopolymer application technology.”
In the article, Epibiotech is described as having expertise in four key areas:
- Hair papilla cell isolation and cultivation technology.
- Induced pluripotent stem cell manufacturing technology.
- Organoid-based hair follicle cell differentiation technology.
- Gene editing technology.
Also of interest, in August 2022 Epibiotech hired two new staff members to its leadership team. One of them is described as a vice president of the Ministry of Food and Drug Safety. He is an expert in the medical product field and a regulatory approval review specialist.
Other Recent Updates
Epibiotech CEO Sung Jong-hyeok seems to give numerous documented presentations at local conferences, universities and more. Some recent highlights:
- Update: July 12, 2022 — Epibotech human clinical trials to commence with a year. Another article from earlier this month states that a dermal papilla cell pipeline, was completed in June 2022. The company “will apply for an IND in the second half of the year and enter Phase 1 clinical trials in 2023″. Also of note, preclinical work confirmed very good efficacy. One month after transplantation of human dermal papilla cells into pig skin, the number of hairs increased by 40% and the hair thickness by 30%.
- Update: April 29, 2022 — Epibiotech announced on April 29, 2022 that it would expand the EPI-001 autologous DPC (dermal hair papilla cell) therapy to off-the-shelf allogeneic treatment.
- Update: April 21, 2022 — Scientists from Epi Biotech were involved in an interesting new paper. Its conclusion: “Silencing the CXCL12/CXCR4 signaling pathway can be developed for novel hair growth stimulation.”
- Update: October 5, 2021 — Epibiotech has started producing non-clinical samples of its dermal papilla cell therapy EPI-001 at its cell therapy production center.
Epibiotech New Hair Loss Facility
- On June 3rd 2021, Epibiotech and T&R Biotech announced a partnership to develop a hair loss treatment involving pluripotent stem cells. According to T&R Biotech’s CEO:
“Epibiotech’s dermal papilla cell differentiation technology and our pluripotent stem cell line will create a combination with high synergetic effect.”
- On May 31, 2021, Epibiotech and its CEO Jong-Hyuk Sung announced the construction of a new production facility at its Songdo headquarters. This GMP facility will be used to produce “2,000 self-made dermal papilla cell therapy products annually”.
Epibiotech’s platform technology page is all over the place and a bit confusing. It includes dermal papilla cell separation and culture technology as well as gene editing based hair follicle stem cell production.
Note that CEO Jong Hyuk Sung is a professor at Yonsei University. This company was originally founded in 2015.
- On April 30, 2021, Epibiotech signed an agreement with Professor Hyung-beom Kim of Yonsei University to develop a hair loss treatment using gene editing technology.
All of this seems a bit too fast and crazy.
However, as I outlined in my recent post on Han Bio, South Korea is becoming a world center for hair loss cure research. On par with the US and Japan if media articles are to be believed.
Renaming from Stemore
I e-mailed Epibiotech to inquire about their technology and their May 1, 2021 renaming from Stemore. Below is part of the reply (with minor corrections) from Dr. Nahyun Choi, the company’s director of research:
“The name STEMORE, used previously, was based on a focus on research on hair loss using stem cells when the company was founded. However, our company is now researching and developing not only stem cells, but also various hair cells. This includes dermal papilla cells, and an expanded pipeline to low molecular substances and whitening agents. Therefore, the scope of the research and development was extended to hair and skin. So the company name was changed to Epibiotech after the foreword of epidermal cells.”
Epibiotech Pipeline
According to the company’s pipeline page, they are working on three hair loss products.
- The main focus is on EPI-001, the autologous dermal papilla hair cell therapy treatment (with paracrine effect). It is scheduled to enter Phase 2 clinical trials in 2024. They have added EPI-008 in this category too.
- The EPI-002 RIPK1 inhibiter (gel or ointment) will also enter Phase 3 trials in 2024.
- EPI-005 CXCL12 inhibitor.
- EPI-007 Wnt signaling modulator (with paracrine effect). Seems to now have disappeared.
- They previously also showed EPI-003, an oral PGD2 inhibitor product. It was supposed to finish Phase 2 clinical trials in early 2025. Now it seems to be have been removed from the above list.