Around 90-95 percent of people with hair loss suffer from androgenetic alopecia (AGA), also known as male pattern baldness. Just 1-2 percent suffer from alopecia areata (AA), aka patchy hair loss due to an autoimmune problem.
However, in recent years, alopecia areata (and the related alopecia totalis and alopecia universalis) have been in the news almost every week. Most of the coverage tracks miraculous before and after hair growth results via the use of JAK inhibitors. I covered this subject close to ten times a few years ago. More than 300,000 Americans currently live with severe alopecia areata.
FDA Approves Baricitinib for Alopecia Areata
Yesterday, the US FDA approved Olumiant (baricitinib) oral tablets to treat adult patients with severe alopecia areata. This drug is sold by Eli Lilly and Incyte. This once-a-day pill is the first ever approved in-disease systemic treatment for adults with severe alopecia areata (AA). Monthly cost without insurance will be $2,500.
Olumiant is a Janus kinase (JAK) inhibitor that blocks the activity of a family of enzymes. This in turn interferes with the pathway that leads to inflammation in alopecia areata. Note that Olumiant is already approved for treating moderate and severe rheumatoid arthritis. It is available in 4 mg, 2 mg and 1 mg tablets (with the recommended dose for AA being 2 mg per day).
The approval for alopecia areata was based on Lilly’s BRAVE-AA1 and BRAVE-AA2 trials. These were the largest Phase 3 clinical trials ever completed in humans suffering from AA. Both trials were led by Yale University’s Dr. Brett King.
Patients in the Lilly study experienced relatively mild side effects, including slightly increased risk of acne, urinary tract infections and other infections. Those side effects were easily treatable or improved without treatment. Around one-half of patients regrew their hair after one year of treatment.
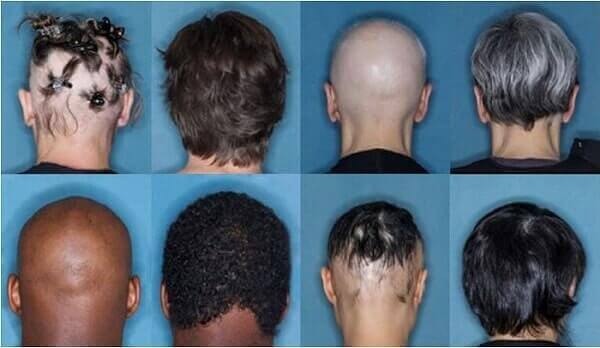
Note that it was Aclaris Therapeutics which originally got the patent rights for the use of baricitinib in treating hair loss in 2017. Earlier this year, I also discussed some phenomenal baricitinib hair growth results. The before and after photos (seen above) are courtesy of Dr. Brett King and his work.
JAK Inhibitors
A number of other JAK inhibitors have shown impressive results in treating alopecia totalis and universalis. Among the more famous of these include ruxolitinib, tofacitinib and decernotinib. See all my past posts covering JAK inhibitors for more details.
Pfizer (Ritlecitinib) and Concert Pharmaceuticals (CTP-543) are also planning to release JAK inhibitors to treat alopecia areata in the near future.
