I have covered cosmetics behemoth Allergan (Ireland) many times on this blog in the past. Earlier today, the company entered into a hair loss product related agreement with Exicure (US) that made major news headlines. I cover this story in more detail at the bottom of this post.
The two spherical nucleic acid (SNA) based products involved are not even in Phase 1 trials yet. Therefore, I am not at all excited about this news. However, it is interesting to see how Allergan is becoming omnipresent in the hair loss world.
Note that Allergan was close to being purchased by Pfizer in 2018, but the deal fell through. In 2019, Allergan was acquired by biopharmaceutical company AbbVie. This was a major takeover that required $30 billion in bond purchases earlier this week.
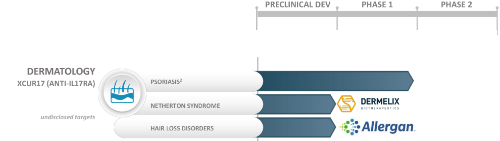
Allergan in the Hair Loss World
Allergan has purchased or partially funded numerous companies that are involved in the search for a new hair loss treatment or cure.
Among these include all of the below, which I have covered in detail on this blog in the past.
- Bimatoprost.
- Setipiprant (acquired in 2015 via Kythera).
- Pelage Pharmaceuticals (acquired in 2019).
- Stemson Therapeutics (partially funded in 2019).
Allergan also owns rights to the Botox brand. Moreover, the company has partnered with Histogen in the skin regeneration sector.
It seems like Allergan is purchasing stakes in hair loss treatments that widely differ in their mechanism of action. i.e., the company is hedging its bets that a miracle could come from many different areas. Dihydrotestosterone (DHT) reduction is clearly passé.
Allergan Funds Exicure’s Hair Loss Product Development
Eerlier today, it was announced that Allergan was providing $25 million in immediate funds to US based biotech company called Exicure. The agreement gives Allergan exclusive licensing to Exicure’s two hair loss drug candidates.
Exicure will be eligible to receive milestone payments of up to $97.5 million per program. Ultimately, if the product is commercialized, Exicure can get further milestone payments of up to $265 million per program.
While I have never heard of Exicure before, the company is apparently working on two different hair loss treatments. Their technology is based on Spherical Nucleic Acid (SNA).
Unfortunately, both hair loss products are yet to commence Phase 1 trials. Allergan’s funding will probably make this initial step more likely.

