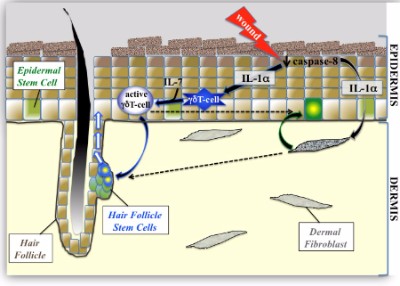
In 2004, Aderans Research Institute filed a patent (granted in 2009) titled “Tissue engineered biomimetic hair follicle graft“. The invention entailed an improved scaffold that would mimic the architecture of the native hair follicle. The ultimate aim for this invention after further improvements would be hair multiplication. However, for a number of reasons, the much hyped Aderans liquidated its research institute in 2013.
In the above patent filing, the most cited author when it comes to reference material was Durham University (UK)’s Dr. Colin Jahoda. To be specific, 10 of his past papers are cited: ranging from this one from 1981 to a 2001 paper on trans-species hair growth induction. The industrious Dr. Jahoda has published numerous other major research papers since 2001, some of which I have covered on this blog in the past.
Biomimetic Engineering of Human Hair
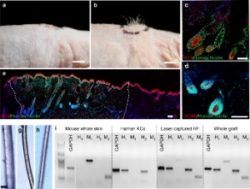
Several days ago, a groundbreaking new research paper was published in Nature Communications. The title of this paper was: “Tissue engineering of human hair follicles using a biomimetic developmental approach”. Very similar to the title of the earlier mentioned patent.
Moreover, one of the main co-authors of this latest 2018 work is Durham University’s Dr. Colin Jahoda. The other authors are all researchers from US-based Columbia University’s Department of Dermatology, led by the renowned Dr. Angela Christiano.
The conclusion of this research is one that should make everyone enthused:
“The ability to regenerate an entire hair follicle from cultured human cells will have a transformative impact on the medical management of different types of alopecia, as well as chronic wounds, which represent major unmet medical needs.”
Note that this latest paper was submitted in May 2018, accepted in October 2018, and finally published in December 2018. So the Jahoda, Christiano et al. team’s current research is at least seven months ahead of what is described in the paper.
3D-Spheroid Cultures to 3D-Printed Molds
I have covered 3D-spheroids, 3D-culturing and related structures and scaffolds (to help brand new hair follicles grow from scratch) numerous times. This area of research has seemed to be the holy grail for scientists trying to succeed at hair multiplication. Just like DHT elimination and restarting Wnt/β-catenin signaling have been the holy grails when it comes to preventing further hair loss and regrowing existing miniaturized hair.
Numerous scientists such as Dr. Colin Jahoda and Dr. Takashi Tsuji have focused on research 3D-spheroids and 3D-culturing of dermal papilla cells to grow new hair follicles for many years. However, in this latest study, it seems like the scientists have turned there focus to 3D-printing (or 3D-bioprinting). They even give the name of the specific 3D printer that they used during this experimentation.
The Jahoda, Christiano et. al team created 3D-printed hair follicle molds as the key component of the experiment. The scientists used a biomimetic approach to generate human hair follicles within human skin constructs (HSCs). They emulated human biology via the 3D organization of cells in the hair follicle micro-environment using 3D-printed molds. The actual paper is very technical.
The authors suggest that in the future, 3D bioprinting technology operating at a single cell resolution may permit the inclusion of many other cell types. This would include stem cells and melanocytes, which would generate hair cycling and pigmented hair follicles.
Some interesting quotes from the paper:
“We recently addressed this issue by 3D-spheroid culture of cells and thereby restored 22% of the hair inductive DPC gene signature. Subsequently, other groups also reported the use of this method to induce HFs in mice, albeit inefficiently. To enhance the efficiency of hair induction properties, in this study, we combined genetic and microenvironmental reprogramming strategies by overexpressing the MR gene Lef-1 in combination with spontaneous DPC spheroid formation in the HSCs, which resulted in 70% success rate of HF formation ex vivo, compared to only 19% with the empty vector-transfected DPCs.”
“Using 3D-printing approaches, our goal is to engineer HFs as follicular units and/or in desired patterns that can be integrated with surgical robots and facilitate effective hair transplantation surgery.”