Every time when I feel like I am done covering Aclaris Therapeutics and the company’s topical JAK inhibitors for treating androgenetic alopecia (AGA) for a few months, some new surprise comes up. The company has not even as yet commenced Phase 1 clinical trials for its AGA product. Yet we keep getting interesting new updates on a regular basis.
Aclaris Therapeutics: Phase 2 Trials for JAK Inhibitors for AGA
This week I got several emails from Aclaris, and they mentioned their continued work and plans for the topical JAK product to treat AGA. Nothing much new in there. However, earlier today, my google alerts for Aclaris gave me a link to this new informative eight page interview with the CEO Dr. Neal Walker and various others. How does “Seeking Alpha” get these interviews before other sites?
In any case, I had to re-register with the above site in order to see the whole interview (which can be set to show up on one page instead of on eight pages). A key quote from the chief scientific officer Dr. Stuart Shanler clearly stood out and necessitated my writing this post:
“We also intend to initiate a Phase 2 open-label trial with ATI-50002 in AGA that is androgenetic alopecia in the first half of 2018.”
If this really does happen as scheduled, it would be superb news. However:
- I am a bit confused about why their topical AGA product in their pipeline is not labeled as ATI-50002, even though the above quote implies as such. It is also worth remembering that just two months ago, Aclaris was granted several patents that implied that a number of different JAK inhibitors (“-tinibs”) could help male pattern hair loss sufferers. So in the end they might perhaps come out with several different topical products for AGA rather than just one.
- It would be useful to know how they can skip Phase 1 clinical trials since their pipeline still shows those as not having even commenced as of today. As many have postulated in the past, since some of these topical JAK inhibitors have already been tested for other uses and by other entities such as Confluence and Columbia University, perhaps Phase 1 trials can be skipped or sped up?
- They plan to conduct open-label trials, which can be a bit biased it seems.
In any event, unless the interview with Mr. Walker et al. was transcribed incorrectly, this is a big development. Moroever, the first half 2018 Phase 2 trial initiation goal comes right from the horse’s mouth.
H/T to “Malcolm” for pushing me to write this post today.
Admin,
Most probably, Aclaris is set to trial Tofacitinib for AGA. As if memory still serves me, this is the jak tested for AA.
This reminds me of when Angela Christiano, during an interview with Kobren, let it slip that jak works for aga, only to quickly re-phrase her statement.
While keeping my feet on the ground, these are really interesting times.
Hi Malcolm, I read that they were testing Tofacitinib for AGA several times in the past, including just earlier this month when I mentioned it in a post.
But it is becoming very hard to track since we went from “JAK” to “topical JAK” to “covalently bound JAK 3 (or was it 2?)” to “soft JAK” etc… and we are also including Confluence and Columbia U patents in there. And they refuse to label their product as Tofacitinib or Ruxolitinib.
Fingers crossed…and definitely interesting times as you say.
Admin,
With regards to your 3 points, I guess that Aclaris can easily push straight to phase 2 with regards to AGA, if the topical consists of either Tofa or Ruxo.
However, as I’ve alays stated, Aclaris, is a fast mover straight to the top, and my gut tells me that they are sitting tight on a gold mine, and they know it.
The reasons for my logic is as follows:
1. Aclaris has bought Angela Christiano’s IP. Yet after receiving the payments (s) she is still on board either as an independent council or otherwise still conducting research,
2. The fact that Aclaris has sought to secure a patent for Tofacitinib administration for AGA, is an indicator that most probably they are vying to test Tofa for AGA.
3. It’s true that Aclaris has purchased the rights to Soft Jaks through Confluence acquisition. However, I have read mentions on your blog that there might be two types of AGA, and who knows?, maybe it’s a fact. Otherwise, it could be a possibility that Aclaris, is set to leave no stone unturned in the quest to cure the common and widespread type of hairloss.
Whilst writing down these points, Angela Christiano’s slip up about jak being a cure for AGA is constantly coming up in the back of my mind.
” As many have postulated in the past, since some of these topical JAK inhibitors have already been tested for other uses and by other companies such as Confluence, perhaps Phase 1 trials can be skipped or sped up?”
I’ve said this before and am not ambitious or curious enough to research myself…perhaps the answer can be found in Minoxidil’s timeline from BP med to AGA topical. I was around for that…yeah, Yoda is that old! :-)
Hello, everyone.
This seems a little out of place and random, but, has anyone tried “Stop and Regrow”?
Gtfo! Haha well that is definitely great news. Looks christiano wants to start the race for aga cure against her boy Cots. Well now it comes down to how well this works. I’m praying for serious regrowth like the AA pictures with Jak. I wonder how long this trial will take? 1 year? Can they skip phase 3?
Interestingly, the Q3 press release on their website does not mention dates for AGA:
https://aclaristherapeuticsinc.gcs-web.com/news-releases/news-release-details/aclaris-therapeutics-reports-third-quarter-2017-financial
The only official AGA information is “Continue to develop another series of topical JAK inhibitors for the treatment of androgenetic alopecia (AGA).”
According to the statement contained in the first paragraph of page 3, Aclaris is set to commence phase 2 for aga.
https://seekingalpha.com/article/4121648-aclaris-therapeutics-acrs-ceo-dr-neal-walker-q3-2017-results-earnings-call-transcript?page=3
Some great news from Hsasson & Wong:
“We are so pleased with our results that we are no longer prescribing oral Finasteride at our clinic “.
“Serum Finasteride levels are reduced by approximately 94% compared with the oral form . Serum DHT levels are reduced in the order of 30% (compared with 70% with oral).” …so are they saying it is half as effective at reducing serum DHT levels in the scalp??…how would this “silicone net” thing even work?
another 5 years ….If I get one hair back each time I heard that; I should be Alec Baldwin.
Seriously. As the crucial alleged dates for Follica and Shiesdo near, suddenly everyone starts hyping OTHER companies. If we are disappointed in 2018/2019/2020, the hype train will start talking up another “in the next five years” company. And the beat goes on…
Conspiracy!!
@admin: LOL. Great news!!!
so true. now people are already talking about follica POSSIBLY coming out in 2020, and shiseido isnt even mentioned anymore. really schizophrenic.
by autumn 2019 no one will even know who is tsuji, lol. but we ll for sure have a lot of promising treatments that should come out in 2023.
If you’re going to make a troll account of my moniker, at least mimic my use of proper grammar, punctuation, and spelling.
I will bet anyone here with monopoly money plus boardwalk and park place that there is not going to be a major breakthrough. Sheseido and replicel are not getting along. Sheseido can just do their own study. Replicel will just jockey for new investors. I mean they can’t even hit deadlines…since we are not suppose to bet with real money…my monopoly mkney tells me that this would be a poor investment and investors see it as that. When you invest you want to know % return and how fast. Dead money is bad money…if replicel was so good…it wouldn’t need to jockey for time and money. Sheseido..if they were smart would go their own way…IF they believed in what they are pitching. Fact is replicel cannot deliver phase 2…on time. The contract could be endangered with the two companies. Lets see I made this post kind of like this a few weeks ago and I am saying it more deliberately now. Nov. 9 2017. I would like to be wrong…that’s what is great about science one can be wrong and if that leads to better things …good. However, I doubt it with replicel.
I thought they hang up between them was Shiseido not sharing their data with Replicel (or the other way around?) Not anything about missing deadlines.
I think it was the other way around. I think replicel didn’t hit phase 2, if I read it properly a month ago.
Hey Admin, I tried posting this earlier a couple of times but it hasn’t gone through, so even if it’s off topic, please bear with me.
Any idea on topical Hydralazine for AGA? I believe Rophe Pharma is starting tests on this… It’s apparently faster and more effective than minox, and it’s also a blood pressure drug…
Hi Admin – bumping this in case you missed it. Sorry, I’m new to commenting on your site so I hope that’s OK. Would be interested to hear your thoughts on this.
Hi Siddharth, I mentioned the CEO and his blog at least twice in the recent past in response to others asking this question. It seems like he is involved in God and Love?
Oh wow. Just wow.
But his personal bigotry/fundamentalism aside, any thoughts on the drug itself? Sorry if you have already answered this, would appreciate it if you could point me to the relevant posts if so.
I ask because I’m hypertensive, and adding another blood pressure medication (in a home brew topical form) doesn’t sound like the worst idea in the world to me, especially if there’s a likelihood of it thickening my hair up significantly.
Is there any link between Aga and age-related hair loss?
Id hate to have aga finally cured only to find that my hair wont grow back because im now in my early 40s :/
Honestly docs now are compounding finasteride topically. I mean…maybe a suggestion. I am younger than you…but maybe if comfortable..buzz the hair. See what you look like to the closest thing being bald without shaving it off. Maybe you may find it isn’t so bad. On the other hand, you might go full beast mode on treatments and not like the look on you. Tbh, everyone talks about the “Rock” being bald. However looks and physique are two different birds. If you took away the Rock’s muscle…is he attractive? Not really. So sadly his physique conjures his looks. Look at say Nick Lackey or ryan Reynolds…looks. The rock ..physique.
What is my point? I think so many ppl to try to justify a point with a non logical approach when thinning comes up and they say oh the “Rock”…Etc. Yeah take Bruce Willis fame and money away and what do you got? The average guy in the corner of a bar. Instead look in the biggest mirror that needs to be looked at. That’s you and your mirror. The man in the mirror can be the biggest opponent.
So what is there for someone in their 40s? How do you feel? The first question can be answered. If you did hypothetical buzz your head…not shave…just buzz…and you don’t mind the look. Then hey…that might be the worst it gets..you have an idea of the worst and might say, “That’s not so bad.” Maybe at that point you might change your risk vs reward on hair treatments. If you did, ton of Trt drs. Testosterone replacement therapy. Mind you …this can accelerate hair loss…but if you want to hit the gym and have add it…that is an option. Again though it’s how you feel about the dome. Trt is not meant to make someone massive…it just brings your testosterone levels back to when you were around 24. However before anyone does anything they should have blood work…idc if it’s propecia….before anyone messes with any hormone they should know their levels to identify the problem and not just treat the symptom…I know ..that’s what we mostly do in medicine treat a symptom However it’s great to look at the picture as a whole. Number 2, to get a baseline. Other hormones can impact other hormones. Example…take propecia it stops conversation of testosterone to dht. Ok. However that testosterone will most likely convert to estrogen. So what did we do? Block a masculine hormone then elevate a feminine hormone. Isn’t gynecomastia a side effect…although rare? Hmmm….could that explain it? Yeah bc hormones are not the say all…it’s also receptors.
So I by all means don’t replace medical advice…but there are things you can do….but what do you feel like? Also how is the biggest opponent…the man in the mirror?
GL mate.
Admin, Although talking about the topical for eyebrows (I think the same logic applies to the AGA trial) Neal Walker’s comment on page 5 of the interview is possibly responsive to your query about “Why Open Label”, when he says “I think on your second question, on the Topical that has been initiated and the whole key there was is we want to demonstrate efficacy on the topical formulation I think in the quickest manner…………….we designed it as open-label, so we can get the quickest read-out, show that our topical works”. This certainly demonstrates a desire to move quickly but it may also be an indication of their level of confidence that it works at a level that is so visibly obvious that it doesn’t need a placebo or other comparative.
Good catch I missed that due to skimming through a lot of the non-AGA content.
If this actually gets started in the earlier part of the first half of 2018 (feb or march), then i think thats a great sign. It would show they are confident and have all their ducks in a row.
Unlike, Follica, Histogen and brontzu
I really hope something good comes along soon, going norwood 3 with full frontal baldness incoming at 18 isn’t ideal
Start finasteride asap. My biggest regret is not starting it when I was early 20s when I was a norwood 3 and waiting 15 years.
I am now on it, but after 2 hair transplants I am still a norwood 5.
I did not bother earlier because there was news of hair loss cures coming out in the next 2 years. 15 years later; nothing.
Yes we understand more about hair loss now than we did a mere few years ago but we must also accept the possibility that a cure may be decades away, or even outside of your lifetime.
@Scott: Sorry…but no.
I’ve been on minoxidil 5% for about a month and 2% for 3 months before that and actually it already made a visible difference which is encouraging (right temple looking the same as 1 and a half year ago and the left one which is worse is filling in slightly) but if things go sour quick I might have to consider finasteride.. Even though I really wouldn’t want to mess with it.
Things will go sour very quick and even quicker after a year or so if you only use minox and don’t take an alpha reductase inhibitor asap
Yup. Minox helps keep more hairs in the growth phase but it actually increase 5a reductase which most ppl seem to ignore.
Soon new treatments will come out! GREAT BLOG!
Go Aclaris! Go JAK! Go nasa_rs!
Great Work Admin – Truly a great day for those suffering from AGA. You do not plan to start Phase 2 unless the product WORKS!
We still have a way to go but we have something very positive to follow with real hope.
Thanks for the Notice Admin. Maybe you were right all along 2020. And, I have constantly professed that jak would work. I knew it not from a guess but logical deduction from people who grew hair the Beno guys and campfire older man.
Something real to cheer about.
awesome! Im so PUMPED.
maybe the multiple JAKs and the 3D model angela christiano innovation 5 years ago was about rapid experimentation of multiple variants of drugs might be somehow be one reason to explain the variants.
also if i remember clearly JAK inhibitors worked REALLY fast, like weeks. Maybe that might play into the trials because there isn’t a 12-24 month wait like shiseido or fin etc. Especially in regard to ACLARIS mentioning japan earlier, this is potentially an out of the blue game changer.
Hi admin , any news from the 10th world congress for hair research?
OMG WAIT ACLARIS PHASE 2 = MARKET IN JAPAN RIGHT?
https://globenewswire.com/news-release/2017/10/24/1152293/0/en/Aclaris-Therapeutics-Announces-the-Allowance-of-a-U-S-Patent-Application-and-Issuance-of-a-Japanese-Patent-Covering-Tofacitinib-for-Treating-Hair-Loss-Disorders.html
Does typing in all caps make you feel important?
https://www.hairlosscure2020.com/aclaris-therapeutics-granted-two-patents-covering-jak-inhibitors-for-treating-hair-loss-disorders/
HOW DID YOU KNOW?
At this point, it’s hard to get remotely excited about something with such an incredibly long road of trials still to conquer. The five year wait thing is a cruel joke sometimes but for JAK inhibitors, that’s far too optimistic. I’d say at least 7-9 is more realistic. Without true breakthroughs in the field in that time, save for a few super-responders to finasteride, everyone on this forum and every other hairloss forum will be far beyond saving. This truly is a hopeless and exhausting cycle. FML
Progress!.. It’s all we can hope for. Great news. Thanks Admin. Let’s hope for a 100% response rate. And let’s hope they stay on schedule.
Let me ask this.
Since they are possibly skipping phase 1, all thats being bypassed is the safety trials, correct?
Is it possible that they are still unsure about this working for AGA, hence the phase 2 trial? Or would they have to be pretty darn certain it works before moving to phase 2?
They are getting patents on this see my texts below. Taking time and money they must know something.
Or maybe they are registering patents to gather interest, then announce good results, get lots of stock investment and then fizzle out and research ends?
Although not directly related to hair loss – I thought this was pretty cool. Also, I’ve always viewed advancements of skin rejuvenation with burn victims or others is also an indirect advancement for hair loss sufferers:
https://www.sciencedaily.com/releases/2017/11/171108151607.htm
Read that today too and right away though of Polairytyte (“COOL”).
https://www.wired.com/story/scientists-save-a-kid-by-growing-a-whole-new-skin-for-him/
“And that tissue developed all the things skin should have — hair follicles and sweat and oil glands”.
In September, the United States Patent and Trademark Office (USPTO) issued U.S. Patent No. 9,737,469 and U.S. Patent No. 9,730,877, and the Japan Patent Office issued Japanese Letters Patent No. 6212107. These patents are directed to methods related to the use and administration of certain JAK inhibitors for treating hair loss disorders. These newly issued patents are owned by The Trustees of Columbia University in the City of New York and exclusively licensed to Aclaris.
• U.S. Patent No. 9,730,877 covers the use of various JAK inhibitors, including tofacitinib, baricitinib, ruxolitinib and decernotinib, to treat AGA, also known as male/female pattern hair loss. The ‘877 Patent contains 22 claims and expires in November 2031.
• U.S. Patent No. 9,737,469 covers the use of baricitinib for inducing hair growth and for treating hair loss disorders such as AA and AGA. Additional issued claims pertain to methods of using baricitinib to treat particular phenotypes of AA, as well as to treat other hair loss disorders. The ‘469 Patent contains 10 claims and expires in November 2031.
The patents expire in 2031 thus my guess they want to come out with it by 2020 in order to enjoy 11 years of exclusivity.
• U.S. Patent No. 9,737,469 covers the use of baricitinib for inducing hair growth and for treating hair loss disorders such as AA and AGA. Additional issued claims pertain to methods of using baricitinib to treat particular phenotypes of AA, as well as to treat other hair loss disorders. The ‘469 Patent contains 10 claims and expires in November 2031.
• Japanese Letters Patent No. 6212107 covers the use of tofacitinib in a topical composition or as the sole active therapeutic agent in a pharmaceutical composition for inducing hair growth and for treating hair loss disorders, such as AA and AGA. The ‘107 patent issued with 25 claims and expires in March 2033.
In October, Columbia University received a Notice of Allowance from the USPTO for a patent application covering methods of treating a hair-loss disorder, such as AA and AGA, or inducing hair growth, by administering tofacitinib topically or by administering tofacitinib as the sole active ingredient, and treating AGA or inducing hair growth in a subject having AGA by administering tofacitinib. The application was allowed with 66 claims.
Since already drug approval plus they have patents in Japan that expire in 2031 AND that they are doing phase 2 early in 2018.
My guess they come out with it a topical lotion in Japan Late in 2018. Yes I said it.
Drug already fad approved. They have Phase 2 early in 2018. And have patents. Why not come out in late 2018!
This is not a slow moving soaking up funds baloney research group living off research dollars. This is a For a Profit Money Hungry Fast Growing BioTechnology Company with investors demanding sales revenue and profits. Can only get that by coming out with products. Minoxidil is garbage and that company still came out with it.
I say it comes out in Japan late in 2018. The drug has been approved by For Years that’s why they got patents so fast in Japan. And I say I am right.
You guys have been bald too long too pessimistic. Adding, we got the right president. He can’t just use it as that is breaking the law and all Dems are trying to impeach him for anything. Thus once he hears about a drug that grows hair he will push fad to approve ASAP in America.
Regardless, my boldest prediction Ever. I absolutely think it will be available in Japan in late 2018! And what I mean about that is, topical jak for aga in Japan by late 2018!!
All is my opinion folks.
Nasa_rs,
Without going into the merits of your arguments, I have a feeling that Aclaris are definitely on to something.
Once, Angela Christiano had let it slip, that Jak’s work for AGA, but she quickly rephrased the sentence. This could be just a genuine mistake, or otherwise, she gave in to a momentary excitement at the discovery.
Like everyone else on this blog, I am unaware of any knowledge related to aga cures or treatments. However, I still find these Jak inhibitors fascinating, and I’m following closely Aclaris’s strategy.
From a Marketing perspective, Aclaris would ideally wait to launch their treatment for SK, in order to ensure both a return on investment and to instill further solid trust with potential high end investors, in order to aggregate the necessary capital to market an AGA topical across the board from the early stages of product entry.
Sticking with my prediction, late 2018. Nothing to stop them. They must already know it works. This is a big strong reputable firm and now they suddenly and quickly come out with phase 2 as fast as possible. They are gunning for that huge profit.
FDA approved for years. Got patents. Just need proof in phase 2 that an FDA approved drug also works in topical form to treat hairloss. Japan has those expedited rules, and this drug, again, has been approved for years. Slam dunk., if phase 2 works, and out the door later in the year on Otc in Japan with Doctor prescription.
2017 is the last bald year. 2918 bald becomes a choice. Hello Japan. In America late 2919 or 2020.
All you naysayers are wrong and I will be proven right yet again. I even proved dr. Christianity wrong. After she came out and proved it worked for AA hairloss she said she thought it would not work for aga. But I remembered the Beno guys and campfire guy who had aga and who grew hair. Thus I knew there was a way to do it. And when this worked for AA and Christianity described it as strongest hair growth drug ever and that it grew 100% hair on bald AA I knew this was it.
I knew the Beno guys had bad sunburn, like a bruise. Where the drug soaked under the skin layers and caused their experimental arthritis drug to grow hair on them who were aga, and campfire guy too.
Anyways, drug FDA approved, oatents completed, once phase 2 are done. Presto out in Japan late 2018. I’m right.
Late 2018 you full of hair people.
All is my opinion.
Nasa_rs,
Let’s assume for a moment that you’re 100% right. But although I have no knowledge of both Beno and campfire cases, your hypothesis by default implies, that in order for jak to work for aga, scalp abrasion must be done prior to jak topical application.
Malcolm, No. The abrasion worked in those cases BUT if the right solvent (for lack of better words) is used to saturate the HAK product to lower skin levels that should work even better. Since if Jan’s can constantly saturate lower skin levels by using the product every so often then better chance of working.
But your point is correct and helps explain why Jan’s never worked before (except in the above cases) is the need to have it in lower skin levels, and not just applied to the top skin surface.
dear nasa
don´t forget tsuji team will start human trials in 16 months!!!
Looking foward to tuzui. They better not delay it.
…anyways, has anyone heard anything from Follica? They are lokely the nearest tp an over the counter, fairly effective, new product.
What makes you think follica will be over the counter? They seem to want to market it as a service performed by dermatologists.
Open label is a good sign… It means they know who’s getting treated and so do the subjects. They might be thinking that the regrowth may be similar to AA treated so they are going all in and are expecting exponential regrowth.. That’s definitely what it seems if they are going the open label route
For those who have missed it I will say it again.
I strongly believe that the JAK’s that are already FDA approved; given patents for Alcaeus already in Japan with expiration in 2031. That jak for aga will be out in Japan by late 2018.
It only makes sense. And for those who say 5-10 years. The JAK’s are already in the pharmacies. And Japan has fast track rules, and for a drug already approved its not going to take long.
A lairs is moving faster than anyone on here ever imagined. Phase 2 in early 2018 – that’s just a few months away. And drug already in pharmacy. Just need to get approval for lotion form. And out the door in Japan late 2018.
My opinion.
Good stuff nasa rs!
So if it does work, ar what point would a companylike this finally reveal a photo showng regrowth? Once they own all the patent rights and are not afraid of another company theying to use a knock off against them. Could we see proof shortly after pbase 2 begain?
Also doesn’t Japan only released regenerative medicine early or is anything fast-tracked after Phase 2?
Who knows when they will show pics. Remember they only care about the $$$’s not in telling the world to get more competitors.
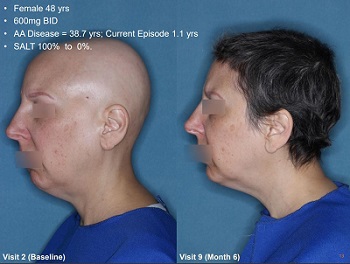
But we have seen AA pics from bald to hippie hair. That’s what it will probably look like. Just look at the AA pics.
Aclaris knows it works. Given, patents already in Japan. Out the door in Japan late 2018. This is a commercial company there is no waiting. Millions have been spent and investors want profits, they do not wait 5-10 years to come out with product already sitting on pharmacy shelves. Japan will give quick approval after phase 2. Jak for aga in Japan 2018.
Nasa_rs,
In my opinion, Aclaris will wait until topical treatment for AA is commercialised,in order to prove beyond doubts that the treatment is safe.
Once AA topical is out, the coMpany would use the product to fast track any product specifically formulated for AGA, into the market.
“Aclaris knows it works”
pictures? are you sure? mmmm
people tried with tofacitinib in others forum without results…
fingers crossed for great news soon
Thanks admin
Fingers crossed
If anyone figures out a way to export this product on the fly workout Tokyo Dr visits they are gong to get rich
I love the enthusiasm Nasa but let’s slow our roll a bit here. They can skip phase 1 because they know it’s safe to use on the scalp since they did this with AA. It may work for AGA but how well? Rogaine well, 100cm2 well, full regrowth? Phase 2 is where they test the effectiveness. Many companies have enter phase 2, Aderans,Intercytex, histogen etc all claiming to work but failed. I agree with you that aclaris believes it works but gut tells me that if it works like the AA, full cure we will never see this product come out. Cures never get released. I feel that this will give us very good regrowth, better than any other treatment but doubt full regrowth. Maybe I say this because I’m just disappointed after 16 years of false hope and lost faith in any treatment for full regrowth but I hope I’m wrong and this crap actually is a breakthrough. Tired of rogsine, fin, scalp massages, etc. Just want my freaking hair back already and not deal with this stupid problem that should have been solved in the 80s. They saw the camp fire guy resukts, why didn’t theu test jak more then? It’s all shady man. Conspiracy haha
Mines, I hat say this but of what you said is completely right. I just know this is it.
I stick with my prediction, late 2018 Alcaeus comes for sale with jak for aga.
Nasa_rs – If you are so confident and it is in the pharmacies already and, presumably you are on here as you have a vested interest, why don’t you obtain this drug and try it yourself?
Obviously if you do, let us all know how it goes please :-)
Not that easy. The pill form is available not the lotion form. Remember lotion form is composed of special solvent type ingredients. Plus pill form way too expensive. They know they will sell millions of lotion tubes thus drop price down very significantly.
Price is not a problem for me. What are these pills, where can I get these pills and what do they cost?
More prostate cancer, more hair loss.
PlncRNA‑1 kills prostate cancer cells
It also grows hair.
Do baldies lack PlncRNA‑1? Thus they lose hair and also get prostate cancer? Because of some interplay between PlncRNA‑1 and androgens/PSAs?
LncRNA PlncRNA‑1 regulates proliferation and differentiation of hair follicle stem cells through TGF‑β1‑mediated Wnt/β‑catenin signal pathway.
Abstract
The present study demonstrated that hair follicle stem cells (HFSc) have multidirectional differentiation potential and participate in skin wound healing processes.
https://www.ncbi.nlm.nih.gov/pubmed/29115537
Nasa there is no way this will be out in pharmacies by end of 2018. They might conclude their phase 2 trials by then. They have to start phase 3 which will probably start in early 2019… if it works. Japan won’t release drugs after phase 2..that’s only for regenerative treatments like stem cells aka sisheido. This will take 3 to 5 years before hitting the market if all goes well. I just want to see a picture of the regrowth for aga!! If they don’t release any pics then something shady is going on because they showed pic of AA and jak.
2020. Right on track
I like when I see Nasa come through. His optimistic outlook give me hope for the future. I hate when I see all of us fighting with each other when we are all looking for the same end result. a full head of hair.
I’m Going To Look Great With A Full Head Of Hair.
We are almost there.
The Browns win a playoff game before a break through hair loss treatment?
Hey admin, can you take an interview with dr. tsuji?
in 15 months organ/riken will start human trials!!!
fingers crossed
Anyone ever used an adenosine product, and if so, which would you recommend for having maximum potency?
I use Adenovital from Shiseido. Can’t say empirically if I’ve gotten results, but I think it’s at least thickened my hair a tiny bit where I spray it. Might be in my head, but I feel like I see darker colored, thicker hairs in those areas, but not a ton. It’s certainly no miracle. However, one great thing is no ‘aging’ side effects like minoxidil has.
Thanks 2cents. This product seems very underwhelming by most ppls accounts. Damn
of all these comments : this is the best I have seen so far
Baldness is the cure, not the disease. I pay $25 to get a hair cut once every 2 months. And I tell the barber the same thing all the time, bald me.
The ultimate cope
Lol
Glad by knowing about jak update..
But some one tell me what is open test?
According to hellouser sheisido has transferred the project to replicel. Anyone else find that unsettling?
replicel is a pink sheet scam
jak lotion don´t work. Maybe is injections nasa???
admin what happen with the interview with Dr. Heather Christofk and Dr. William Lowry??? thenks
Unfortunately, she did not reply to me after I wrote the post and I think she might have changed her mind…perhaps since some of the reader comments are quite immature.
Hi, guys!
Thank you for the article. It really gives us hope. But I have a slightly weird question.
How exactly does this thing work? And more interesting question. How will it work for guys who had hair transplantation or SMP?
Not to be a downer, but Finasteride and Dutasteride both usually do not bring back hair that has been gone for more than a few years. Some exceptional responders out there of course, but not common.