For many years, hair loss sufferers have dreamt of faster and cheaper clinical trials. I think that we are now close to doing so in the US. Some other countries have already streamlined the process, and they will get a competitive edge in new drug development.
For many years, hair loss sufferers have dreamt of faster and cheaper clinical trials. I think that we are now close to doing so in the US. Some other countries have already streamlined the process, and they will get a competitive edge in new drug development.
A large number of companies never develop potentially effective hair growth products due to the prohibitively expensive costs and time involved in rigorous clinical trials. Others end up ceasing trials mid-way through due to the lack of funding.
Table of Contents
- Are prolonged clinical trials on the way out?
- Faster trials in Japan and the UK.
- Secretive China.
- Fast tracking of the mRNA vaccines and its major implications.
- AI and faster clinical trials.
- A list of the numerous mostly failed 10-year+ clinical trials in the hair loss world.
- No financial sense in even beginning clinical trials. Including for dutasteride and oral minoxidil.
- The FDA and its possibly intentional grey zone.
- The Cosmeceutical shortcut.
- The YOLO mentality, social media and increased risk taking.
- The 8 stages of clinical trials (rather than just 3).
Are Prolonged Clinical Trials on the way out?
According to CB Insights:
“Studies estimate that the clinical trial process — where new drugs are tested on patients before the FDA approves them — lasts 9 years.”
I have no doubt at all about this time-frame. For us hair loss sufferers, there have been numerous hair loss companies that have made us wait for 10-15 years while going through extensive three-stage trials.
If everything goes perfectly, the three stages of clinical trials can hypothetically be completed in 5 years. Plus another year for manufacturing, marketing and sales. However, in almost all cases, this does not seem to happen.
Most delays are due to fundraising issues after each stage of the trials. However, many delays just seem like intentional prolongation and stock price manipulation attempts.
Faster Clinical Trials in Japan and the UK
In 2014, I wrote a post on the pioneering initiative made by the Japanese government in fast tracking stem cell technologies in the regenerative medicine space. Their aging population cannot afford to wait ten plus years for new technologies to come to market. The new regulations would allow for significantly faster clinical trials.
A short time after the above news, HairClone’s CEO Paul Kemp told me that they were going to start their trials in the UK due to favorable regulations regarding in-clinic early use.
And just recently in March 2023, it was announced that Britain is set to overhaul clinical trial regulation to fast-track approvals.
“The move comes months after an industry report showed that the number of annual clinical trials started in Britain dropped by 41% between 2017 and 2021, posing a “clear and serious threat” to its reputation as a clinical research destination.”
Secretive China
I also suspect that Kintor Pharmaceutical is seeing faster clinical trial progression in China compared to the US, although I am not certain. The renowned case of the world’s first ever genetically modified babies in China in 2018 makes it clear that shortcuts are easier in that country.
Covid-19 mRNA Vaccines Fast Tracking
The Covid-19 mRNA vaccines were created, evaluated and authorized for emergency use in under a year. While mRNA vaccine research was already going on for a decade, this one year time-frame was insane. Even considering the scale of the global pandemic, I could not believe the speed with which all of this went down.
Even more astounding was how quickly over 60 percent of US residents accepted this new technology into their bodies. In my opinion, the year 2021 represented the turning point in how much risk people and governments were willing to take when it comes to new drug or vaccine development.
The COVID-19 pandemic also set in motion the increasing adoption of decentralized clinical trials. This entails bringing an increasing portion of a trial’s activities to the patient virtually. In contrast to bringing patients to a trial site, as has been the historical norm.
AI, Big Data, Organ-on-a-Chip and Faster Clinical Trials
With artificial intelligence (AI) in the news every single day in 2023, it is no surprise that AI is expected to significantly boost the speed of clinical trials. Last year, I wrote a post on AI and Machine Learning for hair loss drug discovery. When it comes to trials, companies can use AI to rapidly digitize clinical-trial processes so they can complete studies faster. According to a 2022 Deloitte survey:
“76% of respondents are currently investing in AI for clinical development.”
AI can also help in the recruitment, monitoring and retention of trial participants. Including via the use of wearable technologies and via remote videoconferencing and data collection.
Also related is the use of big data and analytics in making clinical trials better and faster. Moreover, organ-on-a-chip technology is coming of age and will boost the speed of drug development in the near future.
10-Year Clinical Trials in the Hair Loss World
In the hair loss world, we have seen many examples of companies taking ten or more years to finish clinical trials. In some cases, they even take ten years to get through Phase 2 trials, and then decide to not move forward.
Among the most well known examples of these frustrating long-term clinical trial scenarios in the hair loss world include the below. With few exceptions, most of these already failed or are likely to fail.
- Cosmo Pharma and its Breezula, which finally began Phase 3 clinical trials in 2023 after 10 years. The company changed its name and ownership structure in between. I am still hopeful that this androgen receptor (AR) targeting product will get released.
- The even slower and increasingly suspect Follica. They must have been at this for almost 15 years by now?
- And don’t even get me started on the 14 years we wasted following Histogen. It surprisingly folded after starting Phase 3 trials; then came back and went backwards to start Phase 1b trials (!); and then re-folded (!!). Hair loss sufferers are perhaps the most gullible group of people on earth.
- Samumed (now Biosplice) was supposed to be the miracle story in the entire world of dermatology. Their valuation levels reached astronomical levels. They even finished Phase 3 trials for their AGA product after a decade of being in the news. Including a cover story in Forbes starring their poker star CEO. Then the hair loss program folded.
- Replicel (Canada) and its partnership issues with the much larger Shiseido (Japan). I still have some hopes that Shiseido will come through with final stage trials. But I would not bet on this technology coming to market anytime soon.
- Dr. Takashi Tsuji and Riken (Japan)’s hair multiplication procedure. This was the single biggest disappointment for me since I started this blog ten years ago. But Dr. Tsuji is not out of the game yet and a previously announced 2020 release has now pushed forward to 2026. By now they should have been in Phase 3 trials, but persistent fundraising issues have delayed Phase 1 human trials.
- After major overpromise and hype, the hair multiplication failures of both Aderans and Incerytex over a decade ago.
- Follicum, which has come back again from the dead. However, I and most other readers suspect that all they will do is disappoint a second time.
- Setipiprant PGD2 antagonist. It was purchased by Kythera in 2015, which in turn was purchased by Allergan, which in turn was purchased by AbbVie. Ultimately, the product never came to market due to disappointing late stage trials. I still cannot get over this audio recording and the fact that it was never removed from the internet.
- Also of note is Aclaris Therapeutics, although they only wasted five years of our time.
- And I hope that Stemson Therapeutics does not turn out to be a dud. They have been in existence since 2017, but have yet to even commence Phase 1 human clinical trials.
No Financial Sense in Getting FDA Approval
In many cases, companies with effective hair loss products do not even try to begin rigorous expensive clinical trials in order to get FDA approval. i.e., they give up even before starting. Even large multinational drug manufacturers are reluctant to go for it, including for the two most popular hair loss products (off-label use) in existence today:
- The most egregious example of this is GSK’s decision to not file for FDA approval to market its enlarged prostate (BPH) reduction drug Avodart (dutasteride) for hair loss. To this day, dutasteride is only approved to treat male pattern hair loss in South Korea and Japan. Even though it has been proven to grow more hair than Propecia (finasteride) in almost all studies that compare the two.
- In all likelihood, blood pressure medicine oral Minoxidil will never undergo clinical trials for hair loss. The profit margins are too low, even though the drug is getting rave reviews from doctors and patients when it comes to its hair growth effects.
The FDA Intentionally Allows this Grey Zone
Below is a great interview of Dr. Jerry Cooley by Dr. Robert Haber. The former elaborates on the slow progress of FDA approvals and the tough job at hand for the FDA. It faces pressures from each side to go slower versus faster.
Dr. Cooley mentions exosomes as being one of those grey zone areas where he is glad to be able to use a reputable product, even though it is not FDA approved. Instead, the manufacturer’s lab is closely checked by the FDA to ensure that all regulations are strictly followed. Reminds me of the recent FDA delays in banning NMN supplements.
Key Dr. Cooley quotes from from 12:27 and 13:00:
“I personally believe that the FDA intentionally allows this grey zone…they actuallly want doctors to do some innovation”.
The Cosmeceutical Route Shortcut
The recent hype surrounding the extremely rapid development and sale of CosmeRNA portends favorably towards new future hair growth products. If a company can get approval to market a new product as a cosmetic, it entirely escapes the clinical trial process.
Even more interesting is the fact that you do not need this approval beyond one country or region to get going. Bioneer is not allowed to sell its product in its home nation of South Korea, but can do so in the UK and EU.
YOLO Mentality and Risk Taking
In my opinion, young people are increasingly becoming risk takers. Part of this is due to the “You Only Live Once” (YOLO) mentality. This is amplified by social media and the emulation of various influencers.
On Instagram, I am amazed to see the countless people who go through various laser, injectable and chemical treatment procedures on their faces. Many are easily influenced by celebrities, TikTok plus YouTube videos, and live real-time treatment demos from clinics.
Even crazier is people who take part in various Group Buys via hair loss forums, Discords and Reddit. They often import drugs and chemicals from sketchy entities in China and Eastern Europe. DIY gene therapy is also a thing.
Where I reside in the US, marijuana legalization has led to tens of thousands of new customers (and bad drivers). Fentanyl overdose deaths hit new records every year in the US. And US retail drug prescriptions are also at a record high. Illegal online drug purchases are probably also hitting new records each year.
I am not trying to make it all sound like doom and gloom. It just seems like people are willing to experiment with what they ingest or inject into their bodies more than ever before.
And I almost forgot. Global hair transplant surgery totals also hit a new record last year. You will probably see the same trend with many other cosmetic procedures. Partly due to the proliferation of Zoom and the work-from-home trend, which has made so many people insecure about their appearance.
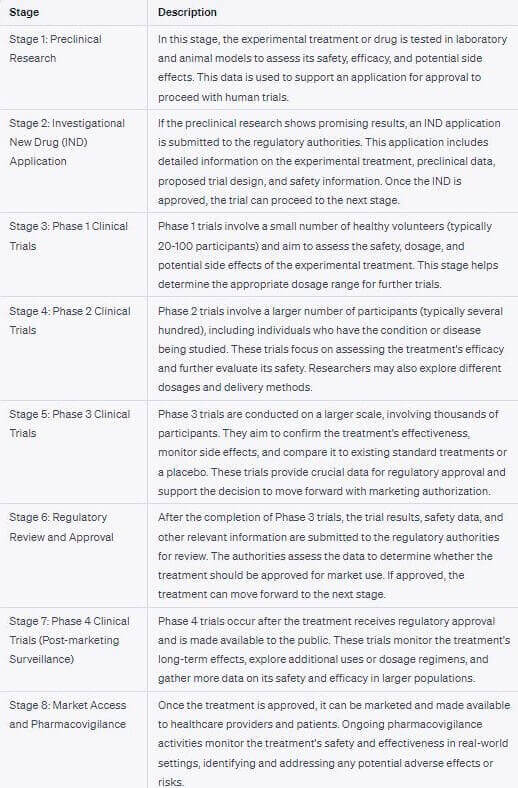
Clinical Trials: In Reality, there are 8 Stages
When I asked ChatGPT to give me the various stages of the clinical trial process, I was expecting around 4-5. Instead, it gave me the below list of 8 steps. I have not even tried to check if all of the below is true to the dot, but it looks accurate.
It is quite clear that the current clinical trial process is antiquated by the standards and expectations of the modern age. And it is also evident that a revamp is in the works in many parts of the world.