 For many years, hair loss sufferers have dreamt of faster and cheaper clinical trials. I think that we are now close to doing so in the US. Some other countries have already streamlined the process, and they will get a competitive edge in new drug development.
For many years, hair loss sufferers have dreamt of faster and cheaper clinical trials. I think that we are now close to doing so in the US. Some other countries have already streamlined the process, and they will get a competitive edge in new drug development.
A large number of companies never develop potentially effective hair growth products due to the prohibitively expensive costs and time involved in rigorous clinical trials. Others end up ceasing trials mid-way through due to the lack of funding.
Table of Contents
- Are prolonged clinical trials on the way out?
- Faster trials in Japan and the UK.
- Secretive China.
- Fast tracking of the mRNA vaccines and its major implications.
- AI and faster clinical trials.
- A list of the numerous mostly failed 10-year+ clinical trials in the hair loss world.
- No financial sense in even beginning clinical trials. Including for dutasteride and oral minoxidil.
- The FDA and its possibly intentional grey zone.
- The Cosmeceutical shortcut.
- The YOLO mentality, social media and increased risk taking.
- The 8 stages of clinical trials (rather than just 3).
Are Prolonged Clinical Trials on the way out?
According to CB Insights:
“Studies estimate that the clinical trial process — where new drugs are tested on patients before the FDA approves them — lasts 9 years.”
I have no doubt at all about this time-frame. For us hair loss sufferers, there have been numerous hair loss companies that have made us wait for 10-15 years while going through extensive three-stage trials.
If everything goes perfectly, the three stages of clinical trials can hypothetically be completed in 5 years. Plus another year for manufacturing, marketing and sales. However, in almost all cases, this does not seem to happen.
Most delays are due to fundraising issues after each stage of the trials. However, many delays just seem like intentional prolongation and stock price manipulation attempts.
Faster Clinical Trials in Japan and the UK
In 2014, I wrote a post on the pioneering initiative made by the Japanese government in fast tracking stem cell technologies in the regenerative medicine space. Their aging population cannot afford to wait ten plus years for new technologies to come to market. The new regulations would allow for significantly faster clinical trials.
A short time after the above news, HairClone’s CEO Paul Kemp told me that they were going to start their trials in the UK due to favorable regulations regarding in-clinic early use.
And just recently in March 2023, it was announced that Britain is set to overhaul clinical trial regulation to fast-track approvals.
“The move comes months after an industry report showed that the number of annual clinical trials started in Britain dropped by 41% between 2017 and 2021, posing a “clear and serious threat” to its reputation as a clinical research destination.”
Secretive China
I also suspect that Kintor Pharmaceutical is seeing faster clinical trial progression in China compared to the US, although I am not certain. The renowned case of the world’s first ever genetically modified babies in China in 2018 makes it clear that shortcuts are easier in that country.
Covid-19 mRNA Vaccines Fast Tracking
The Covid-19 mRNA vaccines were created, evaluated and authorized for emergency use in under a year. While mRNA vaccine research was already going on for a decade, this one year time-frame was insane. Even considering the scale of the global pandemic, I could not believe the speed with which all of this went down.
Even more astounding was how quickly over 60 percent of US residents accepted this new technology into their bodies. In my opinion, the year 2021 represented the turning point in how much risk people and governments were willing to take when it comes to new drug or vaccine development.
The COVID-19 pandemic also set in motion the increasing adoption of decentralized clinical trials. This entails bringing an increasing portion of a trial’s activities to the patient virtually. In contrast to bringing patients to a trial site, as has been the historical norm.
AI, Big Data, Organ-on-a-Chip and Faster Clinical Trials
With artificial intelligence (AI) in the news every single day in 2023, it is no surprise that AI is expected to significantly boost the speed of clinical trials. Last year, I wrote a post on AI and Machine Learning for hair loss drug discovery. When it comes to trials, companies can use AI to rapidly digitize clinical-trial processes so they can complete studies faster. According to a 2022 Deloitte survey:
“76% of respondents are currently investing in AI for clinical development.”
AI can also help in the recruitment, monitoring and retention of trial participants. Including via the use of wearable technologies and via remote videoconferencing and data collection.
Also related is the use of big data and analytics in making clinical trials better and faster. Moreover, organ-on-a-chip technology is coming of age and will boost the speed of drug development in the near future.
10-Year Clinical Trials in the Hair Loss World
In the hair loss world, we have seen many examples of companies taking ten or more years to finish clinical trials. In some cases, they even take ten years to get through Phase 2 trials, and then decide to not move forward.
Among the most well known examples of these frustrating long-term clinical trial scenarios in the hair loss world include the below. With few exceptions, most of these already failed or are likely to fail.
- Cosmo Pharma and its Breezula, which finally began Phase 3 clinical trials in 2023 after 10 years. The company changed its name and ownership structure in between. I am still hopeful that this androgen receptor (AR) targeting product will get released.
- The even slower and increasingly suspect Follica. They must have been at this for almost 15 years by now?
- And don’t even get me started on the 14 years we wasted following Histogen. It surprisingly folded after starting Phase 3 trials; then came back and went backwards to start Phase 1b trials (!); and then re-folded (!!). Hair loss sufferers are perhaps the most gullible group of people on earth.
- Samumed (now Biosplice) was supposed to be the miracle story in the entire world of dermatology. Their valuation levels reached astronomical levels. They even finished Phase 3 trials for their AGA product after a decade of being in the news. Including a cover story in Forbes starring their poker star CEO. Then the hair loss program folded.
- Replicel (Canada) and its partnership issues with the much larger Shiseido (Japan). I still have some hopes that Shiseido will come through with final stage trials. But I would not bet on this technology coming to market anytime soon.
- Dr. Takashi Tsuji and Riken (Japan)’s hair multiplication procedure. This was the single biggest disappointment for me since I started this blog ten years ago. But Dr. Tsuji is not out of the game yet and a previously announced 2020 release has now pushed forward to 2026. By now they should have been in Phase 3 trials, but persistent fundraising issues have delayed Phase 1 human trials.
- After major overpromise and hype, the hair multiplication failures of both Aderans and Incerytex over a decade ago.
- Follicum, which has come back again from the dead. However, I and most other readers suspect that all they will do is disappoint a second time.
- Setipiprant PGD2 antagonist. It was purchased by Kythera in 2015, which in turn was purchased by Allergan, which in turn was purchased by AbbVie. Ultimately, the product never came to market due to disappointing late stage trials. I still cannot get over this audio recording and the fact that it was never removed from the internet.
- Also of note is Aclaris Therapeutics, although they only wasted five years of our time.
- And I hope that Stemson Therapeutics does not turn out to be a dud. They have been in existence since 2017, but have yet to even commence Phase 1 human clinical trials.
No Financial Sense in Getting FDA Approval
In many cases, companies with effective hair loss products do not even try to begin rigorous expensive clinical trials in order to get FDA approval. i.e., they give up even before starting. Even large multinational drug manufacturers are reluctant to go for it, including for the two most popular hair loss products (off-label use) in existence today:
- The most egregious example of this is GSK’s decision to not file for FDA approval to market its enlarged prostate (BPH) reduction drug Avodart (dutasteride) for hair loss. To this day, dutasteride is only approved to treat male pattern hair loss in South Korea and Japan. Even though it has been proven to grow more hair than Propecia (finasteride) in almost all studies that compare the two.
- In all likelihood, blood pressure medicine oral Minoxidil will never undergo clinical trials for hair loss. The profit margins are too low, even though the drug is getting rave reviews from doctors and patients when it comes to its hair growth effects.
The FDA Intentionally Allows this Grey Zone
Below is a great interview of Dr. Jerry Cooley by Dr. Robert Haber. The former elaborates on the slow progress of FDA approvals and the tough job at hand for the FDA. It faces pressures from each side to go slower versus faster.
Dr. Cooley mentions exosomes as being one of those grey zone areas where he is glad to be able to use a reputable product, even though it is not FDA approved. Instead, the manufacturer’s lab is closely checked by the FDA to ensure that all regulations are strictly followed. Reminds me of the recent FDA delays in banning NMN supplements.
Key Dr. Cooley quotes from from 12:27 and 13:00:
“I personally believe that the FDA intentionally allows this grey zone…they actuallly want doctors to do some innovation”.
The Cosmeceutical Route Shortcut
The recent hype surrounding the extremely rapid development and sale of CosmeRNA portends favorably towards new future hair growth products. If a company can get approval to market a new product as a cosmetic, it entirely escapes the clinical trial process.
Even more interesting is the fact that you do not need this approval beyond one country or region to get going. Bioneer is not allowed to sell its product in its home nation of South Korea, but can do so in the UK and EU.
YOLO Mentality and Risk Taking
In my opinion, young people are increasingly becoming risk takers. Part of this is due to the “You Only Live Once” (YOLO) mentality. This is amplified by social media and the emulation of various influencers.
On Instagram, I am amazed to see the countless people who go through various laser, injectable and chemical treatment procedures on their faces. Many are easily influenced by celebrities, TikTok plus YouTube videos, and live real-time treatment demos from clinics.
Even crazier is people who take part in various Group Buys via hair loss forums, Discords and Reddit. They often import drugs and chemicals from sketchy entities in China and Eastern Europe. DIY gene therapy is also a thing.
Where I reside in the US, marijuana legalization has led to tens of thousands of new customers (and bad drivers). Fentanyl overdose deaths hit new records every year in the US. And US retail drug prescriptions are also at a record high. Illegal online drug purchases are probably also hitting new records each year.
I am not trying to make it all sound like doom and gloom. It just seems like people are willing to experiment with what they ingest or inject into their bodies more than ever before.
And I almost forgot. Global hair transplant surgery totals also hit a new record last year. You will probably see the same trend with many other cosmetic procedures. Partly due to the proliferation of Zoom and the work-from-home trend, which has made so many people insecure about their appearance.
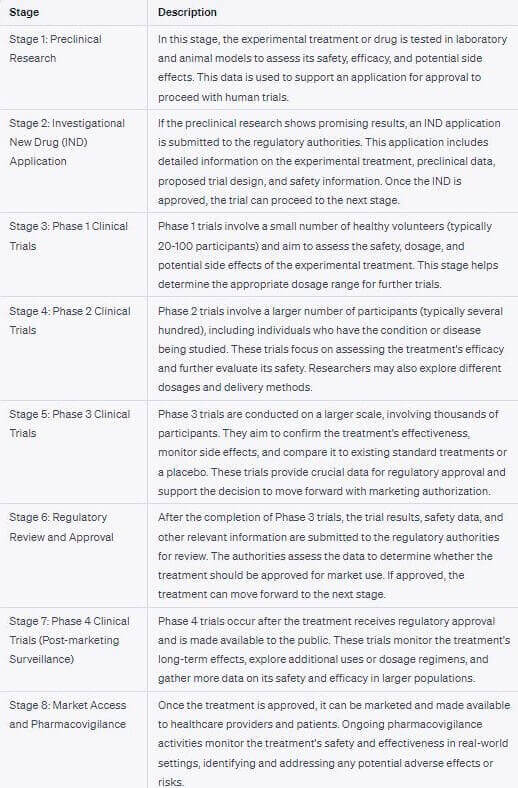
Clinical Trials: In Reality, there are 8 Stages
When I asked ChatGPT to give me the various stages of the clinical trial process, I was expecting around 4-5. Instead, it gave me the below list of 8 steps. I have not even tried to check if all of the below is true to the dot, but it looks accurate.
It is quite clear that the current clinical trial process is antiquated by the standards and expectations of the modern age. And it is also evident that a revamp is in the works in many parts of the world.
We will have virtual clinical trials simulating millions of humans for drug testing in 2035 or after. They will be 1000x cheaper and will be done in weeks/months rather than a decade. Now the problem is the FDA.
Haha, that would be very interesting and perhaps worthwhile. The challenge would be generalizability in that case; to what extent do results in virtual humans translate to actual humans? Kinda like how results in mice translate to humans.
Clinical trials are not always the main problem. Vast majority of companies has scum products.
Hair loss is far more complicated disease than we might think.
I agree Bryan, but whether the product is good or bad, the trial process shouldn’t take as long as it does. If it’s bad, the trial will prove it.
But we’re not just talking about hair loss drugs (though on this site that’s what we’re most interested in – myself included). Speeding up this process will help for other drugs and illnesses as well.
I’m glad they’re finally realizing it. I understand why we have rules and regulations (and I’m grateful for them – safety first) but processes should be analyzed every few years to determine if they’re all still needed and if changes can or should be made. Basically, screw the old adage of “if it ain’t broke don’t fix it.” Some things aren’t exactly broke (per se) but aren’t exactly a well oiled machine either and could use some added efficiencies, changed or even a total overhaul.
Voluntary announcement – successful completion of phase II clinical trial of KX-826 for treatment of AGA in the United States.
Pretty dissapointment results from Kintor.
Too much hype for nothing. Let’s stick to big 3 and get back in 10 years from now.
I still don’t hold much hope for faster clinical trials. We have had fast track in Japan for some years now and still nothing particularly good has come for us as a result.
Admin this report is brilliant it deserves to be in people magazine! Kintor is just another finasteride though.
Thanks. I got my once a decade compliment from Marc!
Appreciate all the effort you put into this admin. 5 gold stars from ol Summy here. As a footnote: I’ve said this a thousand times before…if it ain’t on the shelf, it doesn’t exist – (yet). These company’s remind me of little sea turtles all scrambling to the shoreline, desperate to make it before being snatched up by swooping gulls (ie, other company’s). Then the odds of them surviving in the ocean (ie, the market) are very small. Only a few ever make it.
I think someone has put something in my coffee?!
CosmeRNA bucked the trend! Maybe Kintor will too. Though both unlikely to be better than Finasteride.
A shot of crucifix in your coffee, maybe Summy?
For sure mate. When I start rambling about sea turtles of all things, and then comparing them to the hair loss industry – I think a little Crucifixion mixed in with the coffee beans is well in order.
Excellent piece admin. It’s a shame what appears have happened with follica, although part of me hopes that they’ve bought puretech out and will shortly be announcing plans for coming to market.
Still, if niostem’s figures are real (they said on their Indiegogo comments that they’re publishing them), then it’s problem solved basically and all these AR pathway meds are dead in the water.
Guys, you must understand that the clinical trials are not the main problem. Poor perfomance by the companies in pre-clinical trials are the main problem.
Last decade we learned a lot about the mechanism behind Androgenic Alopecia and structure of hair follicles. Just to remind you that 15 years ago we didn’t even know how to properly isolate dermal papilla cells in mice. We are long way from credible solution for hair loss. That is reality. Stem cells/iPSCs are very promising technology, but still very expensive and robust, and need to overcome some limitations like robust and cheap manufacturing, cheap materials, control the quality of stem cells. There are many new technologies on the way in different areas of science, but they are still far away from clinical applications.
At least ten companies that I/we followed for most of the past 10 years. Without lengthy clinical trials, I would have half the content on this blog.
90% of companies that you covered on this blog dissapeared after 1 or 2 years. What all they have in common is fancy website, fancy team, fancy scientific words, bald CEOs, no before/after photos.
Other 10% of companies that you are covering got results similar or worse than Finasteride in Phase I .
Reality is that we must stick to big 3 in next 10 years.
Way off. I even listed 10 long-term ones in this very post and there are more. You think I covered another 90 plus companies that disappeared after 1 or 2 years?
Admin is there a way to turn off comments from people who just parrot the same nonsense over and over and over again? It’s just wasting time and there’s no new input from these characters not quite sure why they stick around forums and start moaning if they’re filled with that much despair.
Unfortunately not. We get one of those people every few months it seems. I will disallow repetitive comments from this latest guy. I noticed that he made the bald CEOs comment in 4 prior comments.
Sounds good
All of these companies failed all 50 of them the past 23 yrs. Nothing will work ….sorry. get an fue and pray big 3 lasts…it’s just reality.
It’s ridiculous that during Covid drugs for all human beings were tested for only one month and a hair drug must be tested for 10 years.
Everything over 3 years testing is not acceptable..
It is very strange that in 20 years we didn’t see 1 picture before/after from all companies.
And they have bald CEOs.
We live in happy times that nobody can sell you scam treatments anymore.
New scientific paper by the guys from Epibiotech about their pig trials. Check it:
https://onlinelibrary.wiley.com/doi/10.1111/exd.14795
Good find Bryan! We need someone to break this down for all us please.
Add Stemson Therapeutics to the list. They’ve been at it since 2018 and seem to be going nowhere. They report no new news other than hires to their team or management. They’ve been doing pig trials starting the beginning of 2021 and haven’t reported their findings. Their CEO has no clue when anything will get off the ground and has no interest in catering to anyone who isn’t a news maker. Probably will be a pre-clinical company until they fold like all the rest of them. People come and go from this outfit.
Another likely dud is DNovo. They grew hair on human skin grafted onto a mouse in 2020 and since then nothing to report. They too started in 2018.
Then hair was actually cloned in 2015 and grew on human scalps but not in the right direction. Since then we haven’t been able to solve this? WTF? We have biodegradable scaffolding now, so why not try it again in this way so the hairs can grow in the proper direction?
Of course we don’t want to discourage people from trying but sick and tired of all the news and false claims that hair loss has been solved with no workable solutions.
How many more years of this crap will be going on? Will it be 30 years in the future before this is figured out? All this technology we have today but can’t grow hair properly on humans once it’s lost. Sheesh! At this rate humans will colonize Pluto before we get a hair loss cure.
Stemson literally had a seminar where they want to commence human trials next year…
Where did you get this information?
Admin Edit: I updated the link to archive.
https://web.archive.org/web/20221126233142/https://wxpress.wuxiapptec.com/geoff-hamilton-co-founder-ceo-stemson-therapeutics/
It was on this website but the page looks like its been taken down. Trust it, they wouldn’t be continuously hiring new employees if they were a failing biotech company. Just like in life you can’t be too optimistic or too pessimistic, you gotta see things for what they are. Stemson is looking like they’re on the right track so let’s wait and see what they bring to the table. I do believe we’ll have something exciting in terms of news 3/4 quarter of this year. But I’d personally be more excited for Epibiotech considering they are doing clinical trials this year and have a faster clinical process (estimate market release date 2026/2027). Plus Stemson has said they will consider Japan/UK/South Korea for clinical trials where the process will be a lot faster compared to the USA.
https://www.scienceboard.net/index.aspx?sec=cgt&sub=def&pag=dis&itemId=4909 over here geoff says a couple of years is when they feel ready and a scientist on the inside said that there have been insane results in the lab.
https://www.businesswire.com/news/home/20230201005196/en/Kevin-D%E2%80%99Amour-PhD-Joins-Stemson-Therapeutics-as-Chief-Scientific-Officer
https://www.linkedin.com/posts/allan-dovigi-aa962389_regenerativemedicine-folliculogenesis-ipsc-activity-7039449699266809856-H0hu
I never heard any of this. Do you have a link to this information you could provide?
But I have provided multiple links in the comments I previously made?
Added Stemson, with a caveat. Have covered them in many posts and even interviewed Dr. Terskikh in the past, but they are moving way too slowly.
The real “cure“ will be drug that will stop the hair loss at very early stage and give you some regrowth, so you can maintance your hair for years and years without side effects. And it will be cheap to buy it. That is the perfect solution and younger generations will have it in next 10 years.
Most of the guys who are visiting this website are in advanced stage of AGA, so for them “cure“ is some treatment that will give them full head of hairs. That solution is maybe 15 years at least away for ordinary Joe. Even it is out on the market it will take more than a decade to come to your dermatology doctor. Stem cell/iPSCs therapies will require highly trained doctors/professionals. All those technologies will be new and very complicated, so limited number of doctors and clinics will perform it. And that means only limited number of patients will get it next decade. So, pills are far less complicated solution for hair loss, but all treatments involving pills, topicals, injections will have very limited results for most of the people.
Can’t we ask Joe Rogan to talk to the c.e.o of Dnovo or stemsons therapeutics or junji Fukuda or Dr tsuji if he’s not to busy doing witchcraft ?
OT here: Triple Hair of Canada had been planning Phase III trials (US included) for its Minox + Fin + Latanoprost combo. Their Phase III trial had been delayed…and delayed. I’m not sure if it’s still on for 2023, but they are now selling their product at an online pharmacy as a compound. They mention the hair might become darker….I’m trying to remember if latanoporst was one of the compounds that was found to darken grey hair.
There is a study about latanoprost and hair repigmentation after 3 years treatment. What’s the online pharmacy called where they are currently selling?
HairStim can also add Latanoprost. See bottom of this post:
https://www.hairlosscure2020.com/topical-finasteride/
Per a comment from Yoda, Formula 82D (made by the same company as Formula 82F that I listed in the above link) can also include Latanoprost.
I suspect that a few of these topical finasteride compounders can add Latanoprost into the mix if desired.
Hey admin, Great post! A bit off-topic, but have you thought of renaming the website to Hairlosscure2030? ;)
That is the most common recommendation for improvement that I get for this site :-)
Well, judging by how things are progressing it looks more and more likely that we’ll have some decent treatments by the end of this decade. They might not be real cures (for that we may have to wait til 2040, if we’re lucky), but much better treatments that the stuff we’ve got right now.
So would you do it? It’s not bothering me at all, I am just curious. :)
No, I am going to be done and dusted before 2030! And I do not want to rename the site.
A bit of a mix up Admin, the pharmacy (Anazao Health) I was getting the 82D from had a separate product that I used with Lantaprost in it called Phytosol. This also had minox and spiro in it as well. 82D in the AM/Phytosol in the PM. I quit using their products as they had issues with the 82D coming out of suspension, crystals forming. Not to mention they are very expensive. I went back to the stuff I was using before, less money and similar ingredients. I can tell you guys that Lantaprost or Bimatoprost has done zero for my grey hair.
Thanks for the clarification Yoda.
This is an excellent post and effort Admin.
I am curious if Chinese clinical trials even take more than 5 years?
Interesting recent letter from Shinya Yamanaka. He is the winner of the 2012 Nobel Prize in Physiology or Medicine for generating induced pluripotent stem (iPS) cells.
https://japannews.yomiuri.co.jp/editorial/insights-world/20230407-102052/
“Our endeavor for the medical application of iPS cells is set to enter the second half of the race when the real competition begins. We would like Japan to be at the vanguard of the world in realizing iPS cell-based medicine to help many patients awaiting new treatments.”