Kintor Pharmaceutical (China) is definitely the real deal. They are moving faster than any other company in the history of the hair loss industry. Moreover, they are conducting hair loss trials for:
- Two separate androgen receptor (AR) targeting products: a degrader (GT20029) and an antagonist (KX-826 aka Pyrilutamide).
- Each of them in two countries (China and US).
- Each of them for both males and females with androgenetic alopecia (AGA).
So potentially 2*2*2*3 phases =24 clinical trials (plus any pre-clinical work)! Hence my excuse for this post becoming very disjointed. The newest updates are on top. The latest July 2023 pipeline from their site is shown in the below image:
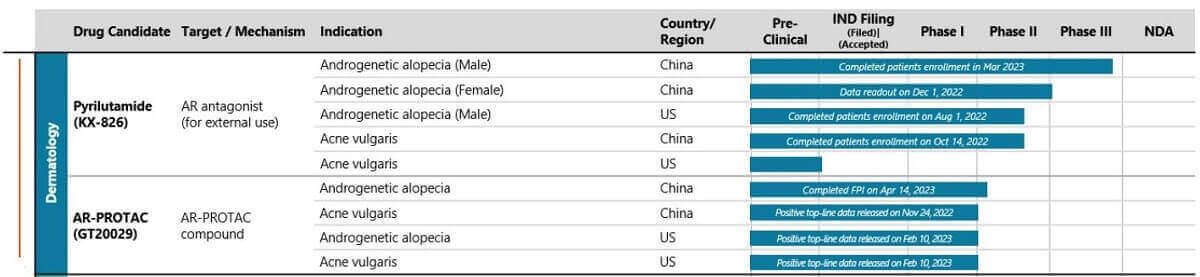
Update: July 19, 2023
Kintor Begins Enrollment for Second Phase III Trial for KX-826 in China
This is insane. If you recall, in March 2023 (see further below), Kintor announced that it had completed enrollment of 740 subjects in China and was beginning Phase III clinical trial of its KX-826 (pyrilutamide) androgenetic alopecia product.
Today, Kintor announced that it has also initiated a long-term safety Phase III trial of KX-826 with the first patient just enrolled. A total of 270 male and female AGA patients will be enrolled to evaluate the long-term safety of topical KX-826 for the treatment of AGA patients in China. The treatment period will last 52 weeks.
At the top of this post, I mentioned that Kintor could conduct 24 potential trials related to its hair loss products (plus preclinical work). It seems like they might be shooting for an all-time world record 32 trials! Most likely, they will not do two Phase III trials in every case. Nevertheless, this company is unlike any other that we have ever seen in our lives. All the stereotypes about Chinese product safety issues and trial shortcuts are clearly not apt in this case.
This is the first time in the history of the hair loss world that a company seems to have absolutely no financial restrictions in conducting clinical trials. That too in two countries (including the stricter US), with no significant delays at any phase of the process thus far.
Update: May 11, 2023
Phase 2 for KX-826 (AR antagonist — Pyrilutamide) Completed in the US
Kintor Pharmaceutical announced that the Phase II clinical trial of its KX-826 molecule for the treatment of male androgenetic alopecia in the US was completed successfully. The results are statistically and clinically meaningful with an increase of about 10 hairs per cm2 after 24 weeks of treatment with 0.5% BID KX-826. The safety profile was also favorable and the company plans to meet the FDA and pursue Phase 3 clinical trials. Note that this same 2 trial was already completed in China last year, with a superior result of a hair count increase of 22.73 hairs per cm2.
Update: April 14, 2023
Phase 2 for GT20029 (AR degrader) Begins in China
Kintor Pharma just completed the first patient enrollment in China for its proteolysis targeting chimera (PROTAC) compound GT20029. The Phase II clinical trial is a multi-center, randomized, double-blind, placebo-controlled study. It will evaluate the efficacy and safety of androgen receptor degrader GT20029 for treating male pattern baldness in Chinese adults. Kintor plans to enroll a total of 180 male AGA patients from 12 centers nationwide.
Update: March 28, 2023
Phase 3 for KX-826 (AR antagonist — Pyrilutamide) Begins in China
Kintor just completed enrollment of 740 subjects in China for its Phase III clinical trial of its KX-826 (pyrilutamide) androgenetic alopecia drug candidate. It expects to release the top-line data in Q4 2023. If the data is positive, the company plans to proceed with the NDA application with the China NMPA.
Update: March 21, 2023
Kintor Pharma Presentation at AAD2023
At the just concluded American Academy of Dermatology Association (AAD) 2023 meeting in the US, there is an interesting update from Kintor Pharma. The renowned Dr. Ken Washenik made a presentation on both of Kintor’s androgenetic alopecia products: pyrilutamide (KX-826) and GT20029. His talk was titled: “The Emerging Potential of Topical Androgen Modulators in Androgenetic Alopecia”.
Besides discussing clinical trial data, Dr. Washenik highlighted the emerging potential of topical androgen receptor (AR) modulators in the treatment of pattern hair loss. Key quote:
“The potential of an effective topical AR blocker without systemic side-effects has long been promising.”
Latest Clinical Trial Status
Per this latest news release (and their pipeline page), Kintor’s latest status of trials (as of March 2023) is as follows:
- Conducting Phase III clinical trials in China of KX-826 for male AGA.
- Conducting Phase II clinical trials in the US of KX-826 for male AGA.
- Planning Phase III clinical trials in China of KX-826 for female AGA.
- Conducting global multi-center Phase III clinical trials of KX-826 for male and female AGA.
- Analyzing results of completed Phase I clinical trials of GT20029 for the treatment of AGA in China and the US.
At the same AAD conference, Dr. Adelaide Hebert also discussed Kintor’s KX-826 for the treatment of acne. It works by competing with dihydrotestosterone (DHT) for binding to ARs. It inhibits gene expression that in turn reduces sebum production and inflammation.
Update: December 1, 2022
Kintor Phase 2 Results for KX-826 in Females
Not surprisingly, yet another positive Kintor update. Their Pyrilutamide (KX-826) phase 2 clinical trial results for females with androgenetic alopecia just got released. And the findings are positive. Total area hair count (TAHC) increased 11.39 hairs per cm2 compared with the placebo group after24 weeks of treatment. It seems like they are going with a dosage of KX-826 5mg (0.5%) once a day, even though twice a day was also tested.
The overall safety profile of Pyrilutamide in females was good, and no participant needed to drop out due to any adverse event. Note that these results, whilst encouraging, are less than half what was seen in men (22.73 hairs per cm2 increase). See further below for the prior updates on the male trials. Both these Phase 2 trial were conducted in China, and Kintor is expected to conduct Phase 3 trials for men and women in China too.
Update: November 24, 2022
Phase 1 Trials for GT20029 a Success
Kintor’s just announced positive top-line results for its Phase I clinical trial of GT20029 for the treatment of androgenetic alopecia and acne. GT20029 is the world’s first topical Proteolysis Targeting Chimera (PROTAC) compound that has completed Phase I clinical trials. It works by degrading the androgen receptor. The topical administration of GT20029 was safe and well-tolerated, with limited systemic exposure. Note that this study was in China.
Later on in February 2023, they got similar results in US Phase 1 trials.
Update: August 29, 2022
Kintor Releases KX-826 Phase 2 Trial Results in Males
Kintor just released the Phase 2 results from China on their website and they are positive. Note that this Pyrilutamide (KX-826) topical hair loss product is an androgen receptor antagonist. In contrast, existing hair loss products Finasteride and Dutasteride are both oral DHT inhibitors.
Key quote:
“The results showed that the KX-826 (0.5%) 5mg BID group demonstrated significant improvement in TAHC as compared with the baseline (increased by 22.73 hairs per cm2; and placebo group (increased by 15.34 hairs per cm2) after 24 weeks of treatment. The recommend phase III dose is determined as KX-826 (0.5%) 5mg BID.”
Note that BID means twice daily. A total of 120 Chinese adult male subjects with a mean age of 35.6 were enrolled in the study. All had a Hamilton-Norwood classification III or higher balding pattern. They were equally divided into four groups:
- KX-826 2.5 mg (0.25% concentration) twice daily.
- KX-826 5 mg (0.5% concentration) once daily.
- KX-826 5 mg (0.5% concentration) twice daily.
- Placebo.
The overall safety profile of KX-826 was good per these results. No serious adverse event (SAE), adverse drug reaction (ADR), or death occurred. After 14 days of topical application, the blood concentration of KX-826 was very low in all dose groups.
Kintor says that it will accelerate the clinical progress of KX-826 and GT20029 in order to:
“Bring more innovative and effective treatment options to the hundreds of millions of people suffering from AGA and acne vulgaris worldwide.”
Update: August 11, 2022
Pyrilutamide Phase 2 Clinical Trial Results
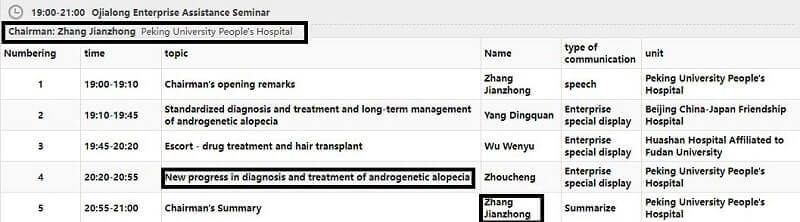
Great news per a Chinese contact. It looks like Kintor may finally present the results of its Phase 2 Chinese trials of Pyrilutamide (KX-826) for hair loss on September 3 at 8:20 pm . The presenter will be Dr. Zhou Cheng from Peking University People’s Hospital (where Kintor’s Phase 3 trials are also being conducted right now).
Note that Kintor is not named in the presentation. However, I noticed that the chairman of this group of presenters (from Peking University) is Dr. Zhang Jianzhong. This man is listed in Kintor’s press releases as the principal investigator. So I decided to update this post as it looks believable now after months of waiting and delays.
The possible presentation (after translation) is titled: “New progress in diagnosis and treatment of androgenetic alopecia.” Please note that this KX-826 androgen receptor antagonist product is unlikely to bring back long lost hair in totally bad areas of the scalp. All I am hoping for is something as good as Dutasteride, but with a different mechanism of action.
Kintor Pharmaceutical Update: Three Clinical Trials
Update: August 9, 2022 — Kintor just completed enrollment of 92 subjects in China for Phase 1 trials of its GT20029 hair loss product. This proprietary Proteolysis Targeting Chimera (AR-PROTAC) product is the world’s first topical androgen receptor degrader compound to enter clinical trials. They will test a gel or tincture per the press release, and results will be finalized at the end of 2022.
Update: August 3, 2022 — Kintor just completed patient enrollment of 121 subjects in its Phase 2 clinical trials of KX-826 (pyrilutamide) in the US. This topical androgen receptor antagonist product will be used to treat androgenetic alopecia.
Update: July 2022 — The China Phase 2 trial results for pyrilutamide for hair loss will occur at the postponed 28th Annual Meeting of the Chinese Society of Dermatology. It runs from August 31-September 4, 2022 in Shenyang.
Updates in April 2022
Some very unique updates in our Kintor channel in the hair loss chat this past week.
- Interesting new Powerpoint presentation.
- Kintor will present the results of its Phase 2 clinical trials (of KX-826, aka Pyrilutamide) for androgenetic alopecia at a “high-profile symposium” in June 2022.
- Some people managed to view a classified Phase 2 results slide that concluded Pyrilutamide’s hair growth effect to be similar to Dutasteride. This would be amazing if true, since Pyrilutamide (targets the AR receptor) is totally different from Dutasteride (targets DHT). Anecdotal reports for now, but seem to be causing a lot of excitement.
- A number of people are starting to report their results from using KX-826 via a Group Buy. I cannot encourage such experimental behavior, but I am following the reports keenly. Here is one I found on Reddit. Best of luck to these pioneers.
Kintor will Start Phase III Trials in China in January 2022
In the latest update from today (November 24th, 2021), Kintor Pharmaceutical announced that it will begin Phase 3 trials in China in January 2022! Update: Now begun.
The company’s IND application for the pivotal study (phase III) of pyrilutamide (KX-826) was cleared by China’s National Medical Products Administration. KX-826 is the first androgen receptor (AR) antagonist to enter phase III clinical trials anywhere in the world.
This is perhaps the best news in the hair loss world so far in 2021.
Update: November 12, 2021 — Kintor Pharma just started Phase II clinical trials in women in China with a first dose of pyrilutamide (KX-826). This trial pertains to androgenetic alopecia in women (i.e., female pattern hair loss).
Update: September 8, 2021 — Kintor Pharma just announced that its Phase II clinical study for KX-826 to treat androgenetic alopecia was a success. Primary endpoint was met. More detailed results will be released later per CEO Dr. Youzhi Tong. Phase III clinical trials in China will commence in the fourth quarter of 2021. Even better, Phase II trials are also currently taking place in the US. And Phase II trials for women will take place in China
Update: July 11, 2021 — The US FDA just approved Kintor’s Phase II clinical trial for pyrilutamide (KX-826) to treat androgenetic alopecia. Note that the company’s trials in China are already mid-way through Phase II per their pipeline page.
They area also working on another hair loss product named GT200029 that is an “AR-PROTAC” compound. Its Phase 1 trials will start in China this month.
April 15, 2021
Kintor Pharma: AR Antagonist and AR Degrader
Earlier today, it was announced that Kintor received approval in China to begin clinical trials for GT20029. This product will be in tincture or gel format, and will be tested for the treatment of androgenetic alopecia and acne.
- The GT20029 product is an androgen receptor degrader (AR Degrader). It is developed using Kintor’s proprietary Proteolysis Targeting Chimera (PROTAC) platform. According to the press release, this is the world’s first topical androgen receptor (AR) compound (AR-PROTAC) to enter clinical trials. GT20029 degrades the AR protein via the E3 ubiquitin ligase pathway. During preclinical studies, GT20029 did not cause any notable side effects or systemic drug accumulation.
- Note that Kintor’s main product for treating male pattern hair loss is KX-826 (Pyrilutamide) and is an androgen receptor antagonist (AR Antagonist). I covered the latter in prior updates to this post if you read till the end. KX-826 is currently in Phase 2 clinical trials in China and in Phase 1 trials in the US.
Note that Cassiopea’s Breezula (Clascoterone) is an AR antagonist that is well ahead of KX-826 when it comes to clinical trial stage. Kintor’s website has a very interesting article discussing both AR antagonist products and hair loss in China in general.
Make sure to also read my related past post on destroying the androgen receptor to reverse hair loss.
Feb 2, 2021
Clinical Trial Status
Kintor’s investigational new drug (IND) application of GT20029 for androgenetic alopecia and acne vulgaris was accepted by the National Medical Products Administration (NMPA) of China.
Kintor has moved forward with its trials faster than any other hair loss company. I am glad to see a Chinese company finally entering the hair loss cure market. Scientific and technological progress seem to happen faster in China than in the west. Hopefully, clinical trials for hair loss products will follow the same pattern.
Side note: In July 2020, Kintor and Applied Biology (US) collaborated on using Proxalutamide for the Treatment of COVID-19. There is a school of thought that suggests anti-androgens could help reduce Coronavirus fatalities. To date, more men have died from the disease then have women.
Below is the pipeline from Kintor’s website:
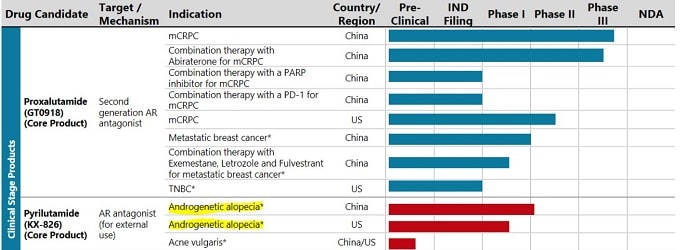
Kintor Pharmaceutical (China) also recently completed the enrollment of 120 patients in its Phase II clinical trials for pyrilutamide for hair loss. See the bottom half of this post for my original discussion on Kintor. Their stock is traded on the Hong Kong Hang Seng Index.
Key quote from CEO Dr. Youzhi Tong:
“We will accelerate the progress of its phase II/III clinical study so as to bring benefits to the people suffering from alopecia as soon as possible.”
May 26, 2020
A new Chinese company named Kintor Pharmaceutical is working on an interesting hair loss drug called pyrilutamide . It is extremely rare to hear about any Chinese company involved in hair loss cure research. Very strange, considering the country’s rapid pace of scientific advancement and massive population. Moreover, Chinese men and women are nowadays balding at much faster rates than in the past.
Update: August 4, 2020 — Phase Ib trials are now complete.
Kintor Pharmaceutical and Hair Loss
Four days ago, China-based Kintor Pharmaceutical (also known as Suzhou Kintor Pharmaceuticals) got significant Chinese media coverage. This interest was related to the company’s prostate cancer, breast cancer and hair loss drugs.
Earlier this month, Kintor Pharmaceutical also had a very successful IPO in Hong Kong.
While the company’s main focus seems to be its prostate cancer and breast cancer drugs, its androgenetic alopecia drug trials are also advancing rapidly. Their main androgen receptor blocking drug candidate is called Pyrilutamide (KX-826) and it is applied to the scalp topically. The company’s Proxalutamide drug slows or stops cancer cell growth by entirely inhibiting androgens.
Pyrilutamide
The one disappointing news is that Kintor aims to take on Johnson & Johnson’s Minoxidil. This could mean that topical Pyrilutamide is unlikely to be much better than Minoxidil. I hope I am wrong. Recently completed phase one trials in China proved that pyrilutamide is safe and causes no major side effects in humans.
Kintor is currently conducting phase 2 clinical trials for pyrilutamide on 160 men in China, and phase 1 trials on 30 men in the US. Phase 3 trials on 600 people in China, the US and Japan are planned for as soon as 2021. I would guess that the US FDA and Japanese PMDA will never accept Phase 2 results from China as any kind of proof to proceed to Phase 3 trials in the US and Japan.
So how can the company proceed so fast in the US and Japan?
Not great that it will compete with Minox, but at least they are targeting the androgen receptor finally.
Maybe they are targeting minox to be seen as the new topical hair loss treatment and not necessarily targeting minox in terms of results effectiveness. Just a thought?
Any word on that shampoo that is suppose to make minox more effective?
Which shampoo, MJones?
It’s Applied Biology – AB-103
https://www.appliedbiology.com/AppliedBiologyPipeline.html#!
Thanks. No pipeline page on there, and website footer says copyright 2018. Otherwise, seems quite interesting.
Jake+Palmer I have a question for you, So if Tsuji transplants the cloned follicles on a human scalp it will take 1 month to see for sure if tumors will form and then if no tumors In the first month it’s a safe treatment? Thanks
That’s a bit of a naive question, 1 month is incredibly short.
Tumorigenesis is very difficult to foresee and needs comprehensive and time consuming testing. First you start with animal testing and then you proceed with highly controlled human testing.
Most cell-based treatments (autologous) seem to be very safe in that respect. But it’s a young technology, hard to tell what happens after 3, 5, 20 years of initial treatment. If humanity wants to advance in medicine, it needs to take certain risks. Japan for example is very liberal in that respect, some would say too liberal…
Yeah for the most part. There have been instances of stem cell injections causing tumors 8 years later. But what’s really happening is, at the moment of injection, the “cancer” cells are simply dividing so slow, that they don’t clump together into a bulge until it gets to a certain point. There’s people who have cancer that don’t even know it. But whenever you inject a cell into the body that is cancerous because theres faults in the dna from replication, they start growing like wildfire immediately. General scans can detect such abnormalities. Tsuji did say he was gonna test the procedure on monkeys before he tested it on humans. In my opinion and I advise everybody on this. I’m not going to get the procedure, no matter how much it costs, until I see a year of results, cancerous screening tests, and results from these tests.
Jake+palmer, thanks for your great recent comments. We definitely need more commentators here who have a good grasp of the science.
For example take a look at this.
https://www.the-scientist.com/news-opinion/japan-approves-ips-cell-therapy-trial-for-spinal-cord-injury-65484
Ips cells “cloned cell” trials have been taking place in Japan since 2015. Mainly for spinal cord injuries, blind people, etc. 80-90% of the time it cures the patients from these problems. And most of these ips trials happen from Riken. Japan is the one of a handful of countries that mandates stem cell screening upon injection. Here in the U.S, it’s kind of a free for all. So though I’m hopeful, I’m still keeping an open eye on long term effects from ips cells.
Thanks Jake+Palmer
Overtime, I would like to see how these teams developing “pre-injection/transplant” screening methods to detect cancerous cell lines. I could see them running PCR to identify key markers (mutated proteins in the cell growth cycle). In general, I only see this technology helping every area of medicine.
Forgot the company name admin. It was posted on FT. It was shampoo that was supposed to be released this year. It was suppose to trigger a mechanism on the scalp so that minox would for non responders. They have other treatments in the pipeline as well.
So maybe we could take KX in combination with Minox to wield better results assuming it actually works?
@Admin I believe Mjones is talking about AB-103 by the company called Applied Biology.
What are the other hairloss drug options to test against then Admin? There are no real topical alternatives AFAIK
Before Antiandrogen by Kintor there will be Cassiopea, Polichem and even Novan will be sooner … Novan SB204 should have finished Phase 3 now and is very safe.
http://www.novan.com/files/8613/7398/9326/Topical_nitric_oxide_local_androgen_therapy.pdf
Anyway many Phase 2 and 3 in the Pipeline now :-)
Very interesting, though they do not list AGA on their pipeline page:
https://novan.com/pipeline/
maybe you could look into this @admin
Yes, I e-mailed them two days ago. No reply as yet.
Phase 3 was finished late 2018:
https://clinicaltrials.gov/ct2/show/NCT02672332
“So how can the company proceed so fast in the US and Japan?”
It won’t need to. China’s middle class out-numbers the entire US population. It’s the largest market in the world. And if it cures baldness, expect medical tourism and the application for Chinese tourist visas to sky-rocket. It won’t need USA or Japanese or European approval.
If second generation oral NSAA’s aren’t curing AGA in the many mCRPC trials that have been conducted, some weak topical AR antagonist hasn’t got a hope in hell.
Its not a weak AR at all though, its quite strong, but as said hundreds of times before No AA will regrow dead hair its just not possible. Biological models castrated before puberty dont regrow hair. Why would any AA regardless of strength?
This isnt an MPB cure its a treatment like finasteride to maintain the hair you have today.
It seems everyone is forgetting this and other prospective anti-AGA topicals will not cure AGA; they will not regrow lost hairs. Rather, they will maintain whatever hair an AGA sufferer has left. Frankly, I wouldn’t be too elated at this Kintor product and others like it. I just hope Tissuse and Tsuji come through before 2025.
I doubt it maybe 2040 and I hope I’m wrong but I used to look at all those doom and gloom guy’s posts saying they’ve been waiting since 1990s and how it’s always 5 years away and thinking “ha we’re like a level 3 civilization now no way will science take that long those guys are just ignorant” but as time has gone on I realized I was ignorant they knew what they were talking about.
Well said H. Unfortunately it’s how it is with our industry. People say tsuji. Tissue etc will be out by 2023 to 2025 lol. I think 2030 to 2040 is a very good time frame. Some rich people might get it earlier but mass use of hair cloning will be around that time frame when its perfected, safe and easily done at a hair clinic.
I’ll be 37 by that time so hopefully I just confront this and get over it i hope we all do or find peace with it somehow even though it sucks.
I think what admin says about an eventual “cure” being a conglomerate of treatments could have legs. Lets say microneedling, and if product X, does up to 15% and product Y does 10%, product Z etc… who knows maybe they could synergize well. Every new treatment/cure will fail…until it doesn’t.
Micro-needling just makes me think of those Edwardian chemists that tried to turn copper into gold. Not inspiration but desperation.
I feel it’s helped me with coverage personally, but everyone’s different.
Microneedling or a dermaroller?
Cassiopea update. Nothing much new:
https://www.cassiopea.com/wp-content/uploads/2020/05/CASSIOPEA-CONSO-Q1-2020.pdf
Any word on whether the PDUFA late August date for Cassiopea’s 1% cream has been delayed? Yesterday, the former FDA commissioner said on CNBC that the virus has caused a case backlog that will delay some PDUFA dates. Hopefully not this one.
Has anyone heard about RU58841? Apparently it works better than Finasteride without the sexual side effects but they claimed it didn’t affect the hormones. Came out in the 90’s. It’s a Topical anti-androgen as well which also claimed to only stop DHT from binding to the receptor in the scalp and also claimed not to be systemic. Although many people on various forums apparently had side effects. Coincidentally it has never completed its full trials presumably because it did go systemic and resulted in side effects. Having an Anti Androgen that powerful that could potentially go systemic would be dangerous.
This leads me to these new Anti-androgens like Breezula or Kintors Drug (Pyrilutamide) which claim not to affect hormones and not be systemic. I wonder if they’d also turn out to go systemic and result in sexual and/mental side effects as well.
Currently RU58841 is available as a “research compound”. I always felt like since it’s available, these new companies could possibly be using it/something similar because RU58841 can be used with various vehicles such as Minoxidil. Also because the name of RU58841 has changed a few times over the years.
For example, Follica has a Micro-needling system that using Minoxidil as well + a compound which in applied in Clinic.
Ofcourse it’s possible that there’s no RU58841 used in any of the new treatments in the pipeline. But definitely something to be wary of regarding the potential of these new topicals going systematic and resulting in similar mental/sexual side effects as Finasteride/Dutasteride/RU58841.
Sonic, you can search for my old post on RU on this blog :-)
Kintor…Here’s hoping?!
Rebranding idea…Hair Loss Cure 2020+
(C,mon Admin, you know it works).
Happy New Year.
I am thinking 20/20 :-)
happy new year, admin.
hairlosscure2021.com domain is still available :p
Thanks for the update admin, great to see a company not messing around. Let’s hope they keep it up.
Another Chinese Company is Hope Medicine with this Bayer Drug. Any news from them?
Also Applied Biology will finish a big Phase 3 Trial with the Kintor drug shortly beginning of this year for Covid. Could this be marketed this year already fast track?
I mentioned the Covid one in the post, but not yet sure about approval. My guess is they have to approve this year or else will become pointless. Not sure about the Bayer status, but will check.
I said it before and I will say it again: we will have this drug from Kintor before Breezula. Finally a company that doesn’t sit on products and waste time
Is this better than minoxidil alone? What the different?
http://minoxistyle.com/MinoxiBoost.html
Off Topic: Is it true that after a certain number of years finasteride stops working? This question has been asked a thousand times, but since there isn’t much on the horizon still, let’s go back and talk about what we already have.
If you ask me, hair cloning was solved back in 2010. I think the quality of hairs produced still remains a challenge. Most researchers are trying hard to mimic the scalp environment to ensure direction and hair thickness correspond to the patients original hair type. That issue wasn’t solved till 2018 when Stemson created micro wells and a lollipop, ” a 3d printed organ mold” for transplantation. the idea is you’d transplant these scaffold in with the cells to make sure they dont separate and you get shitty hairs that either die off or become in grown hairs. If you ask me I still think creating a hair follicle is easy. creating 10,000 is easy. creating 10,000 that have the thickness and curl patten is extremely hard. If you ask me, I could see a cure by 2030, but I think something else will come sooner. I Think a solution could be allogeneic transplant. I think millionaire and billionaire will pay a 100$ a follicle to get donated hair follicles form people that match their hair type, remove the receptors through radiation and implant fresh hair follicles from another person. If we dont have cloning we’ll probably have this by 2030. But I should say this, both of these treatments will most likely be for the wealthy for at least 10 years. Its the same thing as insulin, cost pennies to make but you sell it for thousands of dollars. sad to say but I think people should either start saving in anticipation or wait till 2045 when it becomes economically available.
You seem to be changing your prediction every post, Jake
@jake this has made the most sense more than any other commentator. Histogen is really our only hope and microneedling with minoxidil.
histogen stock is going to 0
Any yet, from yesterday:
https://www.globenewswire.com/news-release/2021/01/05/2153821/0/en/Histogen-Announces-Closing-of-14-0-Million-Upsized-Public-Offering.html
@tom first, timelines change based on liquidity in the market. that’s like saying apple will make the iPhone 10 and it runs out of money 2 years before consumer expectations. Timelines are based on how well a company is funded. Its odd though. Healthcare is the one industry investors aren’t willing to wait 10 years to receive an x multiple on their money. infrastructure, tech, finance, are industries investors are willing to invest for decades to get a return. Your timelines are as good as the companies in the market. All I was trying to say is I firmly believe ips cells as a whole will be used in medicine within the decade given advancing technologies, but I think there will be a premium for a long time. those that think that cloning will be out within the decade are right, but those that think it will be offered to the common man are dead wrong. I dont see it being widespread for decades because nano scale manufacturing of cells and bio degradable scaffolds is really really really expensive. Doing anything on the cell level is expensive. So I say that if your dead set on improving yourself, start saving money
Sei qui che ancora parli ? Non ti sono bastate le tue teorie su tsuji? Parlavi di 2025 e invece? Adesso cosa dici ? La,verità e che non sai nulla ! Sei una persona normale che dice tante cose!
2045! Yikes. I’m out…
Oh stop with the “CHINA OMG SO FAST” thing. It’s BS. I’m an unbiased and independent consultant for s investor (one of out babies just jumped to over 1 billion in evaluation and it’s not an EV company) and I specialize in the innovation sector. In the last 10 years, I didn’t see anything mindblowing or truly interesting coming out of China. And please don’t start with Alibaba or Tencent or Huawei. They didn’t “invent” anything. Their international patents are non-existent, their science papers are mostly absolute garbage (they need to send their students to US universities) and don’t get me even started on the quality issues.
— Recent study from China regarding PROTAC androgen receptor (AR) degrader:
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7498667/
— Seems like these degraders are an increasingly popular area of research to treat prostate cancer:
https://www.nature.com/articles/s41585-019-0248-5
https://www.sciencedirect.com/science/article/pii/S1476558619304865
I’m not impressed! If it’s not hair cloning , then I don’t want to hear about another shampoo or topical treatment! Bye my friend I’ll see you in the 2030s when hair cloning is slated to be a reality !
If it can revive completely smooth bald spots, I’d be thoroughly impressed. I’d like to see a NW 7 become a NW 0.
Haven’t been on this site for quite a while.
Unfortunately the latest developments don’t seem promising at all.
I don’t want to sound sceptic but it looks like there won’t be any new treatment in the near future that works better than avodart or finasteride.
I was optimistic few years ago but now it looks like the situation back in 2013.
To all the new guys here I suggest you start using finasteride or avodart to at least maintain what you already got, get a hair transplant if you want your hairline to look better and don’t rely on dull hopes. Don’t make the same mistake many of the users here did.
I wish all of you a head full of hair.
Godspeed to all of you.
@steve preaching facts!
To those of us who cannot or are not willing to use potent systemic 5AR inhibitors, this is good news.
I have to questions about this:
1. Where did the commentators get the 5 years for GT20029
2. How will KX fare in relation to Breezula? is there any information or results?
This is actually great news. Now let’s see if they post any pics from phase 1 or if the trialist got regrowth. If this can grow tons of hair then we got something great. If it can maintain your hair that will be great for younger mpb folks.
What happened to Breezula?
Kintor is my fav company. They are moving way faster then EVERYONE else. As stated, they are developing two different treatments for pattern loss. They recognize the potential revenue. Breezula does not excite me anymore after learning that 10% of users develop HPA axis challenges. I personally think that most of those that cannot tolerate finasteride will probably not be able to use breezula as well. Again this is my unprofessional opinion. If your endocrine system cant make necessary adjustments to fin then there is a good chance it will fail to adjust to breezula’s steroid.
Nothing good came from China. This will be the first only good thing ( well , second, after the paper in year 105 of this era ) ? I doubt it .
China is going to out-pace the world in so many areas before you realize how dumb your statement is.
Promising. I wish more info was available though. A product soon (ish) but what can or does the product do? Photos? I’m sure it’s all coming but for them to be this close with so little info out….just seems odd. But still, good news. So long as the side effects aren’t too bad and it actually does something. I’m so hesitant to get my hopes up these days.
Excellent news Admin! It is also very exciting, according to the press release, that we will get the Phase II read out from Kintor’s China study sometime within the next 2 and a half months.
Hey all I guess everyone is finished commenting on Kintor. On another note, anyone else notice that those with nice hair often have thick eyebrows? I know there are always exceptions but on average most of the guys I know with solid hair, like I mentioned, have nice thick eyebrows. I would say there is a definite link there. Looking at old pics of my younger self, my eyebrows are not as thick as say when I was 21 years old, so I lost some ground there as well. Thyroid tests have always come back in range so I dont think there is an issue there. Anyone?
I have Brezhnev eyebrows and without fin and minox I would have been fully bald. Same goes for my dad who is fully bald.
If Kintor are legit and the majority of skin-hats can get some hair back without any issues-I imagine they will make billions. Not really that enthused about Breezula tbh, it might be good or just ok? Who knows? Wait a few years, then add in the inevitable set-backs, etc. If you get bored, you can always count how many hair loss companies have flamed out in the meantime. At least Kintor don’t seem to be farting around like some of the dusty old lab-coats at other companies appear to be.
https://www.google.com/amp/s/abcnews.go.com/amp/Technology/wireStory/calls-global-database-human-gene-editing-research-78796382
I’m not really intrested on topicals anymore just hair cloning!
@marc
I’m kind of with you on this. I’m just not convinced a topical can really do what I need done. If I want a full head of relatively thick hair, I feel like it’s gotta be cloning. I mean, I’ll take even a little growth but come on…a topical isn’t going to grow hair from a shiny spot on my head. It’s just not. Might make the small and weak hairs stronger but it’s not growing something from nothing. Only cloning is going to do that.
@james1 exactly, no topical or injectable serum will give the full thick hairline. It’s nonsense to even talk about anything that’s not hair cloning related! Dude I spoke to a British hair loss consultant and he told me hair cloning will probably come out between 2025 and 2028. That’s a long time from now and that’s if he’s right which I pray he is but I doubt it.
Eh, that’s not too far away actually. But the problem is when it first comes out it’ll be insanely expensive. It’s not about when it comes out (when they can finally do it), but when it will be available (and realistic key affordable) for the masses – or at least a good chunk of thr population. Not helpful for most of its 10 mil lol
The thing that really sucks is lets say a treatment that fully works finally comes out. I aint going to jump aboard immediately. That waiting period to see if there are any serious side effects is whats gonna make me go completely bald.
https://www.wnct.com/on-your-side/consumer-watch/class-action-lawsuit-filed-over-hair-care-products-causing-baldness-and-scalp-damage/
I think cloning is gonna take a decade. we can grow hair its just the hair doesn’t look natural, it kinks, curls in the wrong places. if you guys want grass on your head go ahead. tbh I think allogeneic hair transplants will happen if someone puts 10-20 million into finalizing trials. you take someone else’s hair that mates your curl pattern and color, blast it with uv rays to remove dendritic cells so their able to bypass immune cells. now allogeneic hair transplants will be super expensive. picture paying people 1000 for 100 follicles, multiple that by 1000 for 100,000 hairs. yeah a million-2 million for hair. tbh id pay that. hair loss really gets to you, its crazy. but yeah if have every hair transplant doctor have a sample of my hair cross check it to patients and pay patients to get a fraction of their hair, thats where I see this going really. and 100 follicles isn’t alot per person, its 1/10 of an eyebrow, 1 square cm so id imagine people would take 1000 for a 100 hairs for a procedure their already doing
aa25 lets just pray to god that verteporfin will work great and for many more transplants if needed. it would get around hair cloning, and the fda because it already approved.
@woofy 97 verteporfin would have already been tested already. We just want to know about hair cloning! Which won’t happen until after 2025 to 2028 Pfffff that’s a long time from now
Marc we don’t know if veterporfin has been tested yet. Let’s just hope it works because I’m not waiting till 2028 for hair cloning you have to wait years and then when it’s finally out and FDA approved or approved in some other country they will punch you with the outrageous price tag. (even though I don’t care about the price it’s more of piece of mind) but anyways no thanks
I don’t understand the hype around Verteporfin. Where is any evidence whatsoever that applying it after removing from a hair follicle from the back of the head will result in the skin regenerating a new one?
@Anthony
Exactly there’s no proof and verteporfin has been around for a long time. I’m pretty sure someone would have tried it already. Sorry woofy97 but verteporfin isn’t going to help anyone! My apologies if I’m coming off rude but it’s the truth!
I’m no expert, but ‘2025-2028’ and then some! It’s like when someone goes to renovate their home and naively forecasts a reasonable budget, only to see the damn thing blow out to a third, or in some cases, half over the original estimate. Hair cloning sounds great and all, but I imagine it’s light years away? Very happy to be proven wrong tho. Btw: has anybody put any coins in Tsuji’s cup lately?
Dr tsuji isn’t embarrassed he came off completely as a fraud quack scientist/dr. He’s done well never hear from him again except for a new line of conditioner to go with his shampoo, other than that we’re not going to get hair cloning from Dr tsuji. And let’s be honest here who has any faith in Dr junji fukuda, the same Dr who said French fries oils can regrow hair and he even put out a date of 2023 to start human trials doesn’t that sound familiar to what Dr tsuji said in 2018 c’mom give me a break and ukhairclone where are they at I thought they were supposed to be offering stem cell treatments in 3rd quarter of 2021? Just another fraud like histogen and Dr tsuji and Dr junji fukuda we all have to wait for stemson therapeutics I thought they were finished with pig trials there major investor fortunis capital said IN a vimeo video earlier this year that there pig trials were a success and that they were hoping to start human trials later this year but Jan the long time commentator and my balding brother just said they never started it I’m like WTF someone is lying here. But anyways there’s really no hope other than stemson in my humble opinion but I’m right
@Kim yeah between 2025 to 2028 which is almost a decade and then some More easily. Hair cloning is not going to happen until the next decade.
A topical is the real deal ? Sorry, no.
Did you guys see Stemson raised 15 million.
Stemson more funding —
https://www.businesswire.com/news/home/20210715005329/en/Stemson-Therapeutics-Secures-15M-Series-A-Funding-to-Cure-Hair-Loss
Stemson makes moves! I like Alexey T!
It looks like the pig trials haven’t started yet?
@Jan they do not mention pig trials at all, and that vimeo video from fortunis capital said they’ve just finished pig trials in the beginning of this year in there latest news release. Jan you personally told me that they finished pig trials now your saying they never started it
Jan bro why are you saying that they didn’t start pig trials yet. If you’re the one that sent us the link of fortunis capital saying they’ve completed pig trials and they were planning for human trials for later this year, Jan bro if you can please answer me back it would be greatly appreciated Sir Jan.
I cant find it Marc :(
Thanks Jan for replying back.
Kintor results have to be insane for NW5 guys to buy.
Sounds promising! Looking forward to seeing how “statistically significant and clinically meaningful” this turns out to be.
Quantitatively auspicious.
They definitely moved the fastest I mean look at histogen and follica. That deserves props.
Can’t wait to see photos! Moving fast, which is good. Fingers crossed.
Great news. We need a dark horse to come out of nowhere and deliver something significant.
Hey Admin, any updates on the Almirall Topical Finasteride? I was really looking forward to that but it seems it just disappeared… Shouldn’t it be available in Europe by now?
Thanks :)
Just for fun, and even though it seems like the possibility of a real breakthrough is always just a mirage, I’m going to be unobjectively optimistic here: You could make the assumption that if they can make an early call that their Phase II trial was a success, maybe the results are readily apparent from simply visible observation……………in other words, maybe they don’t need to “count hairs” and tally numbers to see that, statistically, they made it over the hump. If that were true, I would think the benefit might be at least a consistent 20% improvement.
Let’s hope this is the case Pinoq. If they can see results without microscope hair count then we got a legit treatment.
Good news indeed. But this is coming from the country that reported 0 covid cases. I’m taking it with a grain of salt. Pics, or it didn’t happen.
China has reported almost 5,000 Covid deaths thus far. South Korea less than 3,000. Japan close to 17,000. Vietnam 14,000. Maybe US totals are miscounted to a much larger extent?
Maybe, but unlikely, unless you were to raise the same quizzical eyebrow in the direction of India, Brazil, the UK, Russia, France, Argentina, Columbia, Italy, Mexico, Peru, Argentina etc?
On a per capita basis, India and Russia have a much lower death rate than the US.
And Africa (with around 50 countries) has a total Covid death count of less than 200,000. In a population of 1.4 billion.
Seems strange that at least 80 percent of the countries in the world have very low per capita Covid deaths. Are they all lying?
In one of the local bars I go to, a long-time sick 85-year-old man had a stroke after a few drinks and died last year. They told his wife that it was due to Covid. She was too tired to try to get a second opinion.
Admin, This just got political very fast. I know of two adults who both had all Covid symptoms and then died of Covid. I don’t think the doctors and nurses are lying on a nationwide scale, and we see some truth given that America has that “free press” thing. I do, however, think foreign governments don’t bother reporting deaths, because they have the same mentality that the former President of the US originally had (image, prestige, and economy are everything.. downplay and deny all threats to that). China, in particular, controls all info and creates their own reality. I’ve been there a few times. There’s a reason their news is government-controlled and their internet is locked down. We shouldn’t be naive about the competitiveness and lack of transparency of foreign governments. The U.S. had a massive vaccine rollout and was mostly masked up (office work from home), yet these other nations report 1-10% of the deaths the U.S. has seen? I highly doubt it.
All I am implying is that by accepting the US numbers, we are indirectly accusing 80 plus percent of the world of lying. Maybe even close to 90 percent if we analyze per capita deaths. Nowhere did I mention politics, so it seems like you made some assumptions? I voted neither Trump nor Biden, but maybe you stereotyped?
https://apnews.com/article/03408313b495c486be3a2e781338259b
“The Department of Health said it had been counting as coronavirus deaths all people who died and tested positive for the disease.”
https://www.factcheck.org/2020/04/hospital-payments-and-the-covid-19-death-count/
“Recent legislation pays hospitals higher Medicare rates for COVID-19 patients and treatment, but there is no evidence of fraudulent reporting.”
— I hope so, especially in diagnosing deaths in those over 80.
Admin, There are enough countries reporting similar numbers to the U.S. such that it helps me trust the U.S. The numbers are scrutinized in so many ways at the county and city level, and it’s my belief that at least some doctors and reporters would find some BS if it existed (this may be my naive outlook). A conspiracy of that size is unhideable in my opinion. The other countries reporting similar numbers to the U.S. are somewhat civilized, like the U.K., France, and even Brazil. So, the countries that I’d expect to be more forthright are the one’s reporting high numbers (despite the bad image). I trust the western countries more than all the rest (many quasi-dictatorships). I say that this is political because choosing which governments to trust or claiming lies and conspiracy tends to be a political belief thing. Anyway, I suppose that none of us know for sure, but I believe reports from doctors and nurses around the country. It’s possible that there’s an agenda to claim Covid deaths and make more money somehow, but I do have a little bit of faith in the medical professionals as a whole (many thousands of doctors and nurses saying what they’re seeing jibes with the numbers). And anecdotally, I know people that sadly didn’t survive it. In summary, good ole France and U.K. have validated everything. That’s half a Eurotrip :-)
Personal experiences definitely make a difference.
The only 1 person that I or my family know relatively well who died from Covid got the Sputnik vaccine a month prior to death. And between me and my family, we likely know at least 2,000 people worldwide. Including in countries with almost no strictly enforced months long lockdowns.
I know 2 that died from covid and some that survived it and can barely breathe from scarred lungs (and dizziness still, six months later).
My mother went to the hospital last week (non covid related) and had to wait 10 hours to be seen – hospital was full of covid patients. No joke, 10 hours. The waiting room was packed and everyone had been waiting for a very long time. Some with serious issues. It was a mess. The staff was stressed out. I don’t think it’s sustainable, long term. It’s not just about deaths. Many that survive it survive it because of hospital treatment but we can’t keep hospitals at 200% capacity forever. Where does that leave those with other injuries and illnesses?
Anyway, let’s hope what’s getting reported on this hair “cure” is legit. Time will tell.
bw. Agreed. As with any company where the goal is to hype things and raise capital (that no one keeps track of), we should always have a healthy skepticism. And China absolutely restricts and controls information. That said, there’s a chance this is legitimate, so it’s good news. Maybe we’ll get some real-world accounts of U.S. participants. Clear before and after photos in identical lighting are the gold standard that seem so incredibly difficult to produce Lol.
Agree. Consistant global pics with head locked into place and using the same camera/lighting to show results is what we want to see. Take it all with a grain of salt otherwise. (Pray the company is not reversing the time-sequence of the hair loss pics too.)
Hi everyone, has anyone heard how it’s going for Dr tsuji fund raising to start human trials. He should have that 4.7 million he was asking for by now don’t you think?
They don’t even have a partner for the trials right? Lets give them…maybe 2 years to find a partner first.
Admin
I will buy PTD-DBM with valproic acid.
This is the treatment of male pattern baldness based on studies .
Anyone in favor of the idea?
Is it a definitive cure for baldness?
Will the hair fall out again after finishing treatment for 3 months or 6 months?
Knowing that PTD-DBM is very expensive.
Please help from forum members.
https://www.lincolnshirelive.co.uk/news/lincoln-news/lincoln-entrepreneurs-launch-life-changing-5903170
Admin please get an appointment / itw with Queen Xiao Rui-Ping to talk about BAY and HMI, she’s the only one in the near future who has the key of our pain in her hands.
Thanks from France.
I have e-mailed her and if she responds I will post on the blog. Please do not ask again as you already did 3 times this week :-)
Will there be any way to purchase pyrilutamide on the gray market before it is approved?
Possibly good news, posted by “SergieS” on our chat:
https://simplywall.st/stocks/hk/pharmaceuticals-biotech/hkg-9939/kintor-pharmaceutical-shares/news/the-non-executive-director-of-kintor-pharmaceutical-limited
This is really good news. I am wondering why the company has not yet disclosed the efficacy data from phase 2.
Ditto!
Definitely suspicious PintoQ. Compounding that is the fact that the FDA is taking a while to approve them for phase 3. There is certainly more to the story than we are seeing at face value.
Will it be possible to import the treatment from China in 2024 or will we have to wait FDA/EMA allow it in 2025-2026 ?
I’d say there won’t be a treatment to import anywhere.
Encouraging! Not telling anyone anything new here, but there’s two ways this can go. 1. Phase III ends in Mediocre, ambiguous claims and results, backed up with mediocre, ambiguous ‘maybe it does something but nah I can’t really tell’ imagery. or 2. Oh my goodness, there really IS a definite reversal of fortunes outcome here, what WAS definitely thin or bald now IS populated with hair and thicker. As always, we hope for scenario two, while staying guarded enough about the likelihood of scenario one for it not to be too disappointing.
Currently I am skeptical of the latter simply because of the lack of transparency. I am getting samumed vibes from this medication. Regardless, we know that simple inhibiting androgens will not lead to hair follicle neogenesis. It will increase hair weight and length though, Androgens are involved in hair miniaturization but are not the cause of male patterned baldness. DHT is actually a very important molecule in formation of scalp hair follicles. It helps make them thick and robust. I am more interested in recent works of inhibiting the PRL receptors found in the hair follicle and this is exciting.
If there aren’t any photos, I’m not getting excited. Not sure how they can expect anyone to get excited without them. We’ve been through too much. When I see (good/solid) photos, I’ll do a dance.
The main redeeming factors for Kintor are that they are moving super fast and working in both China and the US.
Scam companies tend to take almost 10 years from initial press releases to Phase 2 trial completion. Leading us along the whole way while focusing on their stock price movement.
Good points. I’m not saying they’re a scam. But I need photos to get excited. I mean, rogain isn’t a scam but it’s not growing my hair back, you know what I mean? It may help some but not all. It may help all but only increase density by .02%. Who knows? It’s interesting and I’m definitely curious, just not excited yet. They clearly have the funding. Could be great and I hope to heck it is.
Yes, but if its a disappointment or scam, at least we will know just 2-3 years after first hearing of the company:-)
They could have moved much slower and pronged the “hair loss cure (or great treatment) coming” stock speculation for many more years.
Agreed! I do like their speed and the fact that they are moving toward FDA approval means it is a legitimate possibility. But it could be a legitimate treatment at the minimum statistical level on 3 of every 10 people for example or it could be effective on 95% of people at a 15% to 20% level. So I hope they share some exciting evidence soon of how well it appears to work.
There is zero reason to think an AA that works in that way is a scam. We have more evidence for anti-androgens then anything else by far. They absolutely work, and stronger ones will maintain better, but they only will ever maintain.
Great news for younger men or men with hair left.
Not great for high norwoods. Not gonna answer any prayers there
This is not an anti-androgen. And AA is alopecia areata.
Hey admin what are your thoughts on the ethical issues of Kintor? https://www.bmj.com/content/375/bmj.n2819
Also Pfizer:
https://www.bmj.com/content/375/bmj.n2635
https://www.fiercepharma.com/pharma/fda-requests-55-years-to-complete-foia-request-pfizer-s-covid-19-vaccine
Lot of stuff in regards to Covid seems scam-like to me.
Is this supposed to work on patients with mpb too?
Been losing hair for 6 years.
In that time I went from 80% density to 20%. Tried everything and went as far as to use finasterude and avodart together while using minox.
Not only has it been difficult to see any effect but in the last year the bottom has fallen out… I recently stopped filling my scripts but
after a couple months I got motivated again and started using a ketoconazole shampoo while awaiting new meds. In that time my hair loss COMPLETELY stopped. I can barely pull hairs out. Wtf is this?
Had used it before but not consistently.
I know I can chalk it up as just a positive response and I know scalp health is more beneficial than most of us give it credit but how could heavy reductase inhibitors and minox do nothing while this shampoo is completely effective alone?
I’m a diffuse thinner as well and what I have resembles female pattern balding so I was already confused by my case.
Is this bizarre as I think?
It’s been a month I’m starting to hope to see regrowth now that I’ll be back on the meds too but I’m not gonna expect anything
John if you are experiencing true diffuse thinning (FBB) allele than your hairloss will eventually halt prior to complete bald.
It would be nice if the headline was stemson starts phase 3 trial in China. It’s upsetting how slow stemson is going compared to this they have like $22million and they can’t even get the pig trial finished. I don’t want a topical treatment.
Yeah so true…..but its even more sad what riken is doing….or not doing?
Exactly thank you woody 97. Nothing matters but hair cloning!
Best to avoid China. If Xi is foolish enough to invade Taiwan at some point in the next few years then there will be huge disruption to the World economy: Russia/ Ukraine x 10. The UK also has faster testing regulation than the US, which is why there are links to the UK from Stemson I think (?). Personally, I suspect this sort of cell based approach will be the real cure. It may be seven to ten years away, but that in itself is amazing when you consider that the hair follicle is arguably the second most complex organ in the human body.
I have given up on the Western World doing much for hair loss without the usual 10 years of trials (and quitting some where between year 6-10 when the investor money dries up) :-(
What is true diffuse thinning though? And why would that hair loss remain incomplete?
I don’t think this Telogen effluvium because it effects my whole scale consistently which I believe is how TE works for the most part.
Wait what is fbb an acronym for? I don’t see anything about this on the web.
Sorry Jon I meant FPB (female patterned baldness). This is a separate allele that the MPB phenotype . FPB experience hairloss around the whole scalp (primarily on top). It’s most likely x linked so men can get it. It’s a codominant gene and there even if you have one FPB gene you will have some diffuse thinning.
Thank for the response IndianKid.
It just hard to tell if its male or female patterned. but my donor area is going as fast as the rest so it just seems strange.
You seem to have a scientific understanding of the mechanisms here so I’m curious if ur surprised about the main my comments about the effect of Ketoconazale shampoo to completely cease shedding. This was after 6 years of failed efforts. I mean I was losing hundreds of hairs per day while using heavy reductase inhibitors and minox. Now I cant find hairs anywhere after bailing on the meds and using the shampoo out of boredom/curiosity. it’s been effective for over a month and no sign of shedding.
A natural Explanation is that I was very sensitive to the immune response that effects follicles but I still find this insane.
I think the shampoo’s success could be an indicator of sensitivity to the inflammatory response. But this is surely far outside the normal range of what could be considered normal results especially considering previous failed treatments.
Kintor all over the news yesterday due to its Covid treatment proxalutamide. Interim phase 3 trial data will come in December. Proxalutamide is a selective high-affinity silent antagonist of the androgen receptor (AR).
https://www.globaltimes.cn/page/202111/1240061.shtml
How long is this trial going last? Its faster in china right?
Their product is to keep the existing hair right? Its not going to grow anything? But that’s good enough. Would like to throw dut and fin away.
It’s not growing hair? If not, we already have things for that. Heck, that’s nothing special of that’s all it does. I’m sure for some it is, but I’m not going to do backflips over it if all it does is maintain.
My apologies as I know this isn’t the place for it, but wanted to share. I’ve been using Happy Head for a little over two weeks. My hair is definitely a) thicker/fuller and b) super dry. Luckily, with such a low dose of fin in the formula, zero side effects for me. Takes a few months I think to grow, if anything is going to grow (which I’m still skeptical about), but we’ll see.
Thanks a lot for the update James1! I might try topical Dutasteride next year. Have been putting it off and do not trust most such topical products unless produced by companies such as Almirall/Polichem.
I think much of it may be getting the formula right. With Happy Head, Fin is low but there are three ingredients in all. I figure it can hurt to try it for 6 months or so and see. But I’m with you, all these topicals it’s hard to believe any of them. My hair does feel thicker though and I do like that.
@James1 – please keep us posted in HappyHead. I’m 3+ months in trying another product, so I want to wait a few more months. But I’ve got some prostatitis symptoms (had them for years) and I want to get off the pills.
What’s in there and what percentage if I may ask? Once a day application?
Wake up guys, Clinical trial done in China means nothing. China have one of the more less critical institutions in the world. Same with everything came from China, even their cars don’t go to the international crash test that any other car made in the rest of the world does.
Sadly true.
You fool, this company is also having the same trials done in China for the same drug as they are doing in the USA. Do you honestly think that they would try and fool the FDA or perhaps they are confident in their product and are trying to have it approved in the US as well? Pyrilutamide was recently greenlighted by the FDA for phase 2 trials in the US. Even when trying to be negative, think it through logically first.
Yes, but it USA is not even in phase 2! So phase 3 in CHINA means NOTHING!
Hi james+booker, please leave the URL field empty when you make a comment! Thanks.
Yes well Phase 1 is the most critical and most medications don’t get the green light for phase 2. Phase 1 clinical trial, tests the safety, side effects, best dose, and timing of a new treatment. And if you read the article about proxalutamide it showed incredible efficacy with results being released this month. This is nothing BUT good news so far, I don’t understand how you can turn this into a negative when there are moving parts going on in the States too. Things have to go through a process and testing regardless of geography. Plus there have been many cases where drugs that shouldn’t have been approved in the States ended being sold to the public because of the FDA. Just WAIT and see what the results show for phase 2 in the US Lord James+booker.
I agree, I think for once we are dealing with a serious company.
And the stupid example you gave of crash test in China was something like 10 years ago. Have you seen the latest news on crash tests in China? https://paultan.org/2021/09/09/china-carmakers-excel-in-euro-ncap-crash-safety-test hmmm interesting. I wonder how much we can rely on your skepticism and general knowledge?
Please avoid using “stupid” etc.
At the speed with which they approved mRNA vaccines, I suspect there will be much faster (and riskier) experimentation with hair loss treatments going forward. Society in general seems to have become less caring about the long term, and more into “living for today”. Or maybe I am reading too much from social media discussions.
New exosome treatments for hair loss are already risky in comparison to PRP etc. And not FDA approved (or closely monitored depending on year). Add to that the faster rate of trials in Japan, UK and almost certainly China.
Kintor’s stock price is down 85% in the last six months.
Institutions pay much closer attention to this stuff than people realize. They find inside information often well before the public. If the stock doesn’t move up on Monday that should reflect on the truth of this product. Fingers crossed.
The Chinese stock market has been going down as a whole for the past 9 months. The Chinese government has been trying to deflate the bubble for the past year, proactively then letting it burst like it will in the US eventually.
You can’t judge medical success when equities reflect many other variables from a macro perspective.
I’ve traded and followed the stock market for decades. If you have a company that institutions are confident that will outperform, bearish macros will not be able to suppress the stock price. You can find many examples of this over the last 30 years.
FYI — Kintor stock price tripled in past 1 month. But what you say is true when looking at 6 month performance.
Yeah. That was great news for the company. I saw the stock shot up when they announced their Covid drug success. Unfortunately, it’s given quite a bit of those gains back. Yesterday it was down 35%.
I really hope this company can succeed with this new technology. If the news on their hair loss drug is positive and the stock price can hold its gains from the news, I will be more optimistic.
How do these group buys work? Are they obtaining the product legally or is there something sketchy going on?
This wont do better then maintaining, but that is a huge win we should have had 10+ years ago. I mean at least guys can keep their hair safely with this although it will be quite expensive until it goes generic much later. Finasteride honestly just isnt safe to use long term it has too many issues.
Hopefully, the US can approve this quickly and finasteride/dut can go into the dangerous systemic dust bin
Imagine when pyr becomes well adopted and fin sales drop. Merck could actually develop a drug that’s truly indicated for alopecia (not just use a drug like fin for an off label indication).
Hopefully, it actually gets finished, and spurs more competition in the market. If it gets big sales it would really get the train rolling.
I think really the issue is just MPB is hard. Its not lack of effort or trying. Major regrowth would get big sales its just so hard to do.
I don’t think you understand that Merck isn’t profiting a lot of finasteride anymore since the patent is already (long) gone.
It’s a win, and honestly it’s a lot closer than anyone else has gotten (anything meaningful, I mean, that isn’t snake oil). I wish it would do more than maintain, though. We need something that actually helps a little beyond that. Anything beyond that. But I’ll be happy to see something replace fin that doesn’t have side effects.
Hi Admin,
I think I read somewhere that you are taking dutasteride three times per week. Are you taking Finasteride the other days?
I’m asking because I have some side effects with daily dutasteride, but I had side effects with Finasteride too. I was thinking of alternating the two or perhaps go back to finasteride five days a week and dutasteride two.
I realize you aren’t a doctor, but I would appreciate your thoughts, experience, and input. Thank you.
Hi, I just take Dutasteride 2-3 days a week these days.
I will be watching the feedback from the group buy users closely. I hope the product they get is pure and high quality. but even so, I am somewhat worried that extended shipping times will degrade quality. From what I am able to tell from some quick research, Pyrilutamide can remain stable for a few weeks during shipping, but the raw powder needs to be stored in the freezer. And it has a much shorter shelf life in solution. My guess is that the group buy users are generally very experienced and have tried most potential treatments, which probably tends to mean they have already recruited most hairs that were in trouble back to life. This could mask the perceived effectiveness of pyrilutamide. On the other hand, those in the Kintor clinical trial were not on other treatments like dut or fin. So the combo of multiple treatments could potentially exceed the trial results. Fingers crossed!
So any idea when we’ll be getting pictures??
Hi admin – can you explain the difference between
* AR Antagonist
* AR Degrader
* DHT or the mechanism of Dutasteride
Because I thought Dutasteride is an AR Antagonist, but you say differently.
Hi Ben, oral Dutasteride is a systemic inhibitor of the 5α-Reductase enzyme, which in turn reduces dihydrotestosterone (DHT) levels throughout the body. It blocks the action of the 5α-reductase enzymes that convert testosterone into the hair-killing DHT.
Topical Kintor is a localized androgen receptor antagonist. It penetrates the scalp skin to reach the androgen receptors (where it prevents them from interacting with DHT). In more scientific lingo, per wikipedia:
“AR antagonists act by directly binding to and competitively displacing androgens like testosterone and DHT from the AR, thereby preventing them from activating the receptor and mediating their biological effects.”
As far as degrader versus antagonist goes:
https://en.wikipedia.org/wiki/Antiandrogen#Androgen_receptor_antagonists
https://en.wikipedia.org/wiki/Selective_androgen_receptor_degrader
Thanks so much!
Its good but sad at the same time to see a topical AR antagonist finally be finished when we had potential ones 20+ years ago like ascj 9 that never got finished. Things like RU or flutamide. The list of topical AAs and ARs is a mile long, but none of them actually came out officially.
Quite a long time to wait, and I had sides on fin. That stuff really is IMO quite dangerous to use long term. I think it has sides we dont understand that well.
Can anyone help me out? In the article the admin said that Kintor will take on J&J minox. (At the bottom on the page). I don’t understand what they do with the minox. They attack the receptors no?
Would be great if someone can explain me what the actual action of their drug is. Isn’t it comparable with RU?
Hi Georg, it is just based on one of the articles I linked to. In there, it states that Kintor wants to compete with J&J’s Minoxidil for market share. You are correct. No link between the two drugs.
Ah thanks for the Info. So we are happy if its better.
In theory, will this drug be able to regrow hair or is it just for maintenance?
Is there any indication that this won’t go systemic, like topical finasteride does? If it doesn’t stay confined to the scalp, it could also lead to many side effects and could just be another treatment like fin or dut that sensitive individuals (like myself) can’t use.
Interesting article on a new skin rejuvenation technique developed at Cambridge: https://www.bbc.com/news/science-environment-60991675
“The scientists in Cambridge believe that they can do the same thing with other tissues in the body.”
Obviously still decades away, but the science behind it sounds pretty legit.
https://twitter.com/aubreydegrey/status/1512513524815130630
So they are lagging in China for GT20029 trials? Because on Feb 3 of this year, they announced the first US patient had been dosed with GT20029.
http://portalvhds1fxb0jchzgjph.blob.core.windows.net/press-releases-attachments/1372263/HKEX-EPS_20220203_10108141_0.PDF
Interesting, though I did not open the pdf as sometimes it downloads to my phone (which I avoid). In any case, too early a stage for this second product at the moment in both countries.
The fact that a cure could come from China leads me to think of a cure with a relatively low cost. Chinese prefer to sell to everyone at a low price rather than to a few for a very high price. We’ll see …
It’s not a cure, it’s just an antiandrogen…We already have many of those.
its not a cure. Its propecia part 2 that works at a different path in the very complicated MPB process.
Its a preventative treatment largely as any androgen targeted therapy will always be. You cant regrow lots of hair with anti-androgens. We have known this for 50+ years
Admin, do you know what day will the Kintor results be displayed at the congress?
moreover, have you go a ” Chinese mole ” that will be able to attend the congress and provide “inside information” for us? thanks
Since I made that update on the conference, someone on our Discord said there was a second delay… but this person seemed to be messing around. Anyway, it will be this year I am fairly sure.
I think he’s jerking you around since the website of the conference has a countdown that will end on August 31st . Moreover they add a kind of qr code that wasnt here 2 days ago. Have you got a mole that will attend the congress? Thanks.
Thanks. No significant moles, but I have a few slowly growing Dermatosis Papulosa Nigras.
A couple weeks ago, I did see a dermatology conference in China had been delayed until October. So, there may be credence to it being delayed yet again. I forgot the website that lists all the global medical conferences that are upcoming.
They should have finished phase 3 trials in China for KX-826 (pyrilutamide). Right??
Couldn’t find kintor in this schedule.
http://app.incongress.cn/model/rcViewPublic.html?fromWhere=CSD2022
Hairclone ist still alive. I discovered a patent, published last December:
https://patents.justia.com/patent/20210379115
I know a lot of others are not. But I’m quietly optimistic about Hairclone.
Have you heard about the actress Ricki Lake’s story ? She has been battling with androgenic alopecia for 30 years, and recently claimed she regained all of hair thanks to some antihairloss products : https://www.dailymail.co.uk/tvshowbiz/article-11100171/Ricki-Lake-53-shows-incredible-one-year-hair-transformation-photos.html
I dunno, I am skeptical, but what if she tells the truth ??
@hopefullnot. She probably just grew it out like David Beckham does? From shaved and super diffuse looking to (cosmetically at least) full looking. Hard to tell? Maybe she added some thickening cosmetics? Who knows? But I personally wouldn’t believe anything reported from the Daily Mail news “click-bait” service. It’s bottom of the barrel IMO. Similar to McConaughey years ago, probably just an ad for the company. As in the @harklinikken link in her post. $$$. Would love to be proven wrong tho!
Yes…but the thing is the community Women hair loss project also interviewed her, she says she was never paid to advertise those product, i don’t think the manager of that site would interview someone who she feels is not legit…and she came out with androgenic alopecia battle : I pretty much sure she is the first and only women with public exposure who did this (other women did but for other hairloss causes not for andrognic alopecia)… So give. The fact that Aga is way more stigmatizing for women than other hairloss form, that she knows the despair of women who suffer from it…I find it curious that she would be lying about it…the other explanation would be that she reached menaupose recently and agreed to take hormones, and that this why she regained her hair (that also implied that she never tried bcp or Spiro before to treat her hairloss, which seems unlikely to me but wunnos…). It is really weird.
Who really knows? But I hear you. It’s so hard to tell with celebs, bc not many open up about their hair loss and also, bc it’s a private/personal matter and often soul-destroying, you can’t really blame them. It’s just one big question mark at this stage.
A single rule: trust nobody who tells you he manage to grow his hair with cosmetic products :)
Maybe it will be the case one day but for now it’s not, just bs.
Not long ago somebody claimed that in pirylutamide group buys after 5 months nobody had any significant regrow:(
Yesterday i read about the cornea transplant from the tissue taken from Pig and has done a successful transplant with only 8 weeks of immunosuppressant eye drops and thereafter not required. I remember it’s 15 or 18 patients with Indian and Iranian origin and all of them got their eye power back from completely or partial blind patient . It was done in Indian AIIMS hospital. Best thing is that the cornea can be made in infinite numbers and can be stored for 2 years in lab which is not possible for a human extracted cornea.
My point is that if such a level of medical advancement is happening is developing countries then it’s not palpable that hair restoration or regrowth technologies are lagging behind.
New company incoming: Cutaneon
https://www.cutaneon.com/
Renowned Professor Dr. Ralf Paus (founder of Monasterium Lab) founded this new company last year. The approach is completely different to what we heard so far in the world of hair loss: „chemosensory biology“, ion channels and surprisingly the endocannabinoid system.
Dr. Jan Alenfall, founder of Follicum, is a part of the company as is Dr. Kevin McElwee.
Admin, what do you think?
“With an initial focus on cosmeceuticals and nutraceuticals that inhibit or reverse skin aging or control hair growth and pigmentation.”
Am not a big believer in cosmeceuticals and nutraceuticals doing much for hair loss beyond some thickening. Hope to be proven wrong of course.
I agree. If I hear „cosmo- or nutraceutical“ I immediately cringe.
There never was anything from that range of products that helped in a significant way.
But it‘s Ralf Paus, I mean – the godfather of hair research. And the claims are bold:
„dedicated to the development of ground-breaking cosmeceuticals, nutraceuticals, and dermatological drugs“
„generate transformative skin & hair products“
„long-term benefits“
„revolutionize skin & hair product development“
I also cannot rule out that he wants to make his fair share now after decades of academia and he releases some cosmetic products with little use but high prices. Happens a lot unfortunately.
We will see, skepticism is recommended.
@Harryclone this is amazing info.
Hey admin do you know anything about this new treatment?
https://www.prnewswire.com/news-releases/in-the-thick-of-hair-loss-awareness-month-top-hair-doctor-introduces-alma-ted-new-breakthrough-non-invasive-technology-to-improve-hair-growth-301604394.html
No I do not :-)
I’m sorry, anything that Dr. Alan J. Bauman promotes I take with a ton of salt. Only thing that impresses me about that guy is his marketing prowess, he’s never met a “treatment” he won’t put out there. A quick scan of his site, you’ll see laser, supplements, exosomes (I thought the FDA clamped down on this) and my personal favorite…”virtual at home PRP”. He also has salon services, hair systems and anything he can make a buck with.
@Bogdan
Here is the news link.
Deccan Herald: Researchers use cornea implant from pig skin to restore sight in Indian and Iranian patients.
https://www.deccanherald.com/science-and-environment/researchers-use-cornea-implant-from-pig-skin-to-restore-sight-in-indian-and-iranian-patients-1135558.html
Thanks! Great article.
Hi Admin,
Found this article that got posted yesterday – https://scitechdaily.com/new-molecule-discovered-that-strongly-stimulates-hair-growth/
You’re probably across of it but I thought I would post on here and share with everyone.
Yes I wrote a post on it a couple of weeks ago :-)
Can someone explain what the difference is between Kintor’s KX-826 and GT20029?
Do they expect that GT20029 will be an improvement and replacement for KX-826 or is it their intention that both will be used next to eachother in the future? (and if it is possible to use both)
Admin, you mentioned „Inventage Labs“ in your previous post, here‘s the latest news from them:
http://www.biotimes.co.kr/news/articleView.html?idxno=8337
Is a monthly injection an improvement to daily oral intake?
Only if the effects are better and the serum concentration is less, imho.
Hi Ben, they have their own tried and tested injectable delivery mechanism. So I am hopeful it is superior to oral (at least when it comes to localized scalp effect). Could also make no difference of course.
I mentioned Inventage in my past post on finasteride injections. At present, most such finasteride and dutasteride injections are being undertaken at clinics with no unique and proven delivery mechanisms.
What’s the best case scenario for when we will be able to get our hands on this treatment?
I suspect 2024 in China if Phase 3 trial results are favorable. They mention twice in the news article that they will “accelerate” the trials. And Chinese Phase 3 trials already started earlier this year.
Hey Admin, can you explain to me the benefits of this treatment? If you’re a NW3 with diffuse thinning throughout, how will this help?
Thanks.
They only tested on NW3 or higher, which is great. No idea if it will help all or most :-(
Most exciting news in quite a while. I understand that some are disappointed in anything short of the holy grail. However, if this (or the next treatment) actually comes to market it will mark something new in our arsenal since the 1990’s (Propecia). I for one am grateful for what we have (fin/dut/min), what we can learn new (oral, topical, stronger versions) and what will come next!
These results are s… I’m fed up with all the bad news, we need a potion that has to be put only once a month and give us a full head of hair.
This was never going to grow lots of hair, it’s a preventative treatment. I think it’s great news; I hope to stack it with fin for extra insurance and who knows, maybe even grow a few hairs.
Hard to get excited without photos. That bar chart doesn’t really make me do it for me. But I guess it’s only phase 2. I’m sure photos will be available eventually. And glad it’s getting fast tracked. We’ll see.
Guys, be realistic. This is the best we can get in next 5 years.
Stem cells/iPSCs, 3D/4D bioprinting hair follicles, CRISPR therapies are decades far away from practical clinical use.
People with early hair loss can benefit a lot from this kind of treatments on long term. Kintor + Fin + Min can be a real game changer for early hair loss suffers.
I am a female..40. hairloas has been stable for 20 years thank to Spiro 25mg + bcp. However now i am supposed to stop the.bcp….right..what do i have left : my androgen are super low thank to Spiro, close to 0, minox does not work for me….estrogens from the pill are the real deal but now because I am 40 i am supposed to withdraw from estrogens….great….kintor ????
no , within the five next years we will get GT20028 which needs to be applied only a few times per months and will give us full heads of hair.
I don’t know, man – things are always too slowly moving ahead, but the trajectory has changed somehow – products are really entering human trials: Olix is entering phase 1 this year, HopeMed is already in phase 2. Kintor‘s PROTAC is in phase 1, and Kintor are known for ploughing through them phases like a steam train.
The first two had marvellous results in their pre-clinical trials. All 3 have had huge investments into them plus a solid R&D department.
And Epibiotech is expected to hit the market in Korea in 2024 (which is probably too optimistic imho).
It’s as close as never before, but still too far away, as always.
Daaamn. Placebo increased by 15 hairs/cm2? Cancel the study and just give me these Chinese sugar pills.
Placebo increased by around 7 hairs per cm2 and that’s fairly standard , considering hair follicles shed and grow randomly on a human scalp
I’m not taking a pill for the rest of my life for marginal hair growth, because that’s what kintor is it’s another finasteride . we want hair cloning from stemsons therapeutic and junji Fukuda and dnovo . I will not settle for anything less .
Admin check the results one more time because i think on another site i read that 15.34 hairs/cm2 was suposedly the increase of 5mg group compared to placebo but i don’t know maybe i am wrong.
Correct Bogdan.
Treatment grew 22.7 hairs. Placebo “grew” 7.4 hairs per my understanding. See my two replies in this thread (one might be hidden and you will need to click on “Show replies” at the bottom):
https://twitter.com/drdaviperezmeza/status/1564359338629660677
22cm2 is on par with follica…..now which will actually grow 22cm2 terminal thick hairs. That will make a big difference for my diffuse loss. Wonder if it grows hairline hair too. They need pics. Only thing that blows is that it’s a 2x daily topical l…..putting rogaine on 1x a day is annoying. I’ll take whatever that grows hair…..but Bayer seems like a better treatment if they can get it to work on humans. Once a month….thick growth. I’ll pay 500 bucks a month for that if it restores nw1 to nw2 full thickness.
Admin wad this phase 2 trial done in the USA for Kintor?
Can’t it’s been 20 years on propecia and the next potential breakthrough is topical antisdrogen lol…..better than nothing I guess.
Personally, I have pretty much dismissed Follica as a legitimate possibility. It is September and they still haven’t started their Phase 3 trial. I believe Kintor is on its 4th clinical trial of KX-826 (Phase 2,3 & women in China + Phase 2 in US) during the time frame in which Follica has started ZERO clinical trials. I am watching with anticipation for the results of Hope/Bayer, and the start of Scube3. As these are specifically designed to grow hair and not just maintain, there should be pictorial evidence within 6 months of the start of each…………which means we may see something from Dr. Sinclair’s clinical trials by year end.
Check that………….it looks like they haven’t even started recruiting yet for the trial conducted by Dr. Sinclair in Melbourne, Australia: https://www.clincosm.com/trial/androgenetic-alopecia-melbourne-e-hmi-115
I would definitely pay 500 a month for thick hair. Without hesitation.
Yep, better than nothing…I’m not jumping out of my socks, but I’d give it a try if it works (out in the real world) and doesn’t cost a bomb. Interesting to see if it is effective combined too.
admin is there a new article in the waiting line? my self confidence and I need some good news
thanks
I’m so confused. Please help.
Why would my donor area be as thin or thinner than the rest? It’s 10%-15% density everywhere and it isn’t patchy.
I have thin hair – all over. Many don’t have enough donor hair for a hair transplant. It’s unfortunate, but fairly common. The sides usually hold out the longest but they can still go/get thin.
But why would that be? is it female parttern hair loss? Auto immune?
SCUBE3 is 2-3 years from STARTING clinical trials.. prob another 3-5 years after that.
I hate this. I f****** hate this :*(
https://www.hairlosscure2020.com/category/scube3/ look at the update on July 7th it shows when they’re planning for clinical trials
Omg Alexander are you sure about this? If this is true it is really bad info because if you look at kintor they are after 2 clinical trials, the third is ongoing and the release date is stil 2 or 3 years away (and everybody are talking about how fast they are progressing) so if SCUBE3 is starting clinical trials 2-3 years this is bad.
Hey Admin what are your thoughts on SCUBE starting clinical trials, do you think it will really take them 2-3 years?
All companies seem to be slow except for Kintor.
https://dot.la/potential-baldness-cure-2658114684.html
In this article it’s written that any scube3 therapy is 2-3 years away from human trials :( but on a lighter note Amplifica which is Plikus company supposedly has some other hairloss compounds that might be ready for human trials sometime next year.
Great find, thanks Bogdan!
Sounds amazing to me. Series A is planned, new research to be published soon and more candidates than just Scube3 in the pipeline plus trials next year.
I like what I read about Amplifica. And to everybody who is disappointed about timelines: that’s the reality about clinical trials, always been.
Will scube3 grow hair follicles ?
The goal of SCUBE3 is not to grow hair follicles but to reactivate dormant hair follicles. Something similar to restarting a factory.
Is oral Minoxidil approved by the FDA? I haven’t figured it out yet.
Oral Minox is approved by the FDA for high BP, not for hair loss. I have an RX from my doc and use off label. Hope that helps Larry.
Yoda, Do you use 2.5 mg or 5 mg?
Not sure if this has been seen or discussed as I’m not a regular here. But hopefully a positive advancement regarding needling with Rapamycin and Green Tea
https://www.lifespan.io/news/lifespan-news-big-news-for-mice/
Hello admin, the last Chinese female patient for pyrilutamide was enrolled on March 2022: https://en.prnasia.com/releases/global/kintor-pharma-announces-completion-of-patient-enrollment-in-phase-ii-clinical-trial-of-kx-826-in-china-for-the-treatment-of-androgenetic-alopecia-in-female-patients-353590.shtml
This phase lasts 6 months. Do you know when the results will be displayed? thanks
Thanks. Usually, Kintor is great at press release right after any new developments.
good but about 30 years too late for a lot of people. This is something we should have had 30+ or at least 20+ years ago.
Why its take 20+ years to get something off what we knew 40+ years ago? IDK
Is this just to maintain? If so, sorry but whoopty doo. I’m sure great for some but I’m no longer impressed by (or even very interested in) maintain products. They get old, you build up a tolerance, and they no longer work.
If this grows then great, I’m all for it.
Read post again and you will figure out. Hint: Blue font.
Ah, I somehow missed it (just had surgery a few days ago so that be the reason). My apologies.
I have to say this, there has been lots of activity lately. And that’s a good thing. Time will tell if anything comes of it, but there seems to be more movement than last year. Fingers crossed they don’t all fold. I would like all of us to be running our hands through our thick heads of hair by 2025.
No worries, and hope the surgery went well.
They’ll somehow fail like the rest of them….all hype till phase 3 then some ahole big pharma will buy it out and shelf it or they will not release to market for some inconclusive reason. Seen it happen with everything the past 22 years…..I pray I’m wrong but I’m probably not……fue is the only good thing that happened since propecia…..
The formula is already published so there is no sense about buying and not releasing it. Another company would release it then. If phase 3 is successful it would make sense to publish it as soon as possible so that many people use the original product.
At the moment there are so many products in the pipeline – it would be a loss not to hurry up.
Jens.. Good news in your saying the formula is already published. For once, maybe the west can copy China. I hope it’s just available soon somewhere we can all get it.
Even FUE is somewhat overrated. Many I know with thin hair on top have thin on the sides so they can’t even do it. One guy at work got FUE and he has (or had) thick sides but he did 3500 – noticeable but definitely did not fix the problem (I’d still consider him balding, though the 3500 helped). He could get a second FUE but his sides aren’t nearly as thick now so not sure how much he can do. Basically, if you’re shiny bald you waited too long and FUE isn’t going to cover an entirely shiny head.
I think FUE is great if you catch it early (3500 can fix it if there isn’t a ton of loss). But wait 25 years and it’s not such a “cure” anymore.
Why hasn’t stemson therapeutics started human trials yet? There pig and mouse models where a success a long time ago! Why do they have to wait until 2024 ? Junji fukuda/trichoseeds is supposedly starting human trials next year,which I highly doubt junji fukuda will do or be successful at. Kintor, epibiotech,hopemedicene ,mean nothing to me !… Its stemson therapeutics,trichoseeds,and dnovo,and maybe hanbio that were interested in nothing else thank you very much
Okay look, I don’t know why I’ve always been so reluctant to admit it (even here) but I had an FUE about five years ago. Went to a really great doc. I mean one of the best.
Five years later my sides are too thin for another and whatever they tell you a) the rest of your hair will continue to fall out and b) the hair they transplanted isn’t guaranteed to stay – and is now thin as all hell for me. Also, you can feel the bumps where they took the hairs out on your sides (or at least I can).
Would I do it again? I don’t know. The first two years were great. Now not great at all.
And yes, I eat very healthy. Yes, I took minox etc to stop what I could. Yes, he was a great doc. The thing is, 3500 hairs will never cure your baldness. Not even 5k will, more than likely (unless you have very minimal loss). And the hair they move will grow thin. You may lose it. You’ll be close to square one in less than a decade.
Everyone saying it’s the answer – it’s not. Not for most of us anyway. Not bad to cover one small bald spot but 3500-4500 on a shiny head won’t do sh$t. It just won’t. My head wasn’t even shiny and the coverage wasn’t great. And he did a great job. Five years later though and genetics strike again.
Docs tell you a lot of stuff but they’re also salesmen. Keep that in mind.
@James1: Thanks for sharing this. I have read a lot of reviews and nearly nobody is happy 5 years after a hair transplantation. I think hair multiplication might be a cure – especially if they could change the structure of the multiplied hair and make it stronger.
FUE is definitely no cure and there is a lot of bs online about it. I am no fan of plastic surgery at all because your could destroy a lot. If there is a cure in the near future, you will regret it that you messed with your existing hair.
It might be hard but the best thing is to wait right now.
True but many have been waiting 20 years and it may be another 20. I know it feels like it’s right around the corner but people have been saying that for decades. Wait wait wait. Gets old.
But I don’t think there is any cure on the horizon i couldn’t use because i had 3500 hairs transplanted already. Esp if the cure involves growing new hair.
A good doc will always explain the pros and cons before you jump into anything. I’m sure there are reputable HT surgeons out there, however, years ago I had one smarmy bell-end try and sell me a HT without knowing any of my medical history first. He arrived at this decision after “consulting” me for less than two minutes. (He never even left his chair to examine my scalp!) He came across as a cheap used car salesman. I was trying not to laugh at this pelican as he repeatedly praised my ”donor area”. Ha. I severely wanted to donate my fist to the f*#kers jaw instead. I walked out of there wondering how many poor souls had been stooged by the twat.
Totally. My guy was reputable but you’re right, tons of false promises out there. And honestly he can’t know what will happen five years later. Nobody can know. It may look great and you may lose it all.
Kintor Pharmaceutical Limited Announces Positive Top-line U.S. Phase I Trial Results of GT20029:
https://www.marketscreener.com/quote/stock/KINTOR-PHARMACEUTICAL-LIM-111325374/news/Kintor-Pharmaceutical-Limited-Announces-Positive-Top-line-U-S-Phase-I-Trial-Results-of-GT20029-the-42959665/
That is from 5 days ago Meko. I already added it in the post in the GT2009 section (in case you have not read).
Getting these updates is like how I imagine it must feel receiving letters in prison.
I was wondering about the acne side of this. I’ve always had a little bit of issues with some acne on my back and Pyri cleared it right up.
I’ve yet to receive a good explanation why the blood concentration rates of KX-826, that in some cases were documented to be on par with healthy male levels of testosterone, are negligible. Especially when this thing has a much greater binding affinity than test.
KX-826 has no safety mechanism why it shouldn’t go systemic, other than it should reach androgen receptors in follicles before it reaches your blood. But the data definitely isn’t very compelling. No matter how they paint it. On top of that, it seems that the anecdotal evidence from gray market stuff (granted that it is just that, gray market stuff) supports the view that the systemic absorbtion indeed is significant. That stuff seems to be very ridden with side effects.
I find it very hard to be excited about this stuff.
I am looking forward to GT20029.
I agree that the grey market KX-826 doesn’t seem to work properly and also some people claim to have bad side effects. But of course there is the possibility that the grey market stuff isn’t working like the original stuff.
I did grey market. I had bad sides on Fin and had to stop taking it. Pyri I have minimum side effects but def tolerable. It seems to help my hair. I’ve been on it for 4 months. Less shed, some regrowth.
Pyrilutamide 5 month results:
https://www.reddit.com/r/tressless/comments/11xxym5/pyrilutamide_5_months_results_before_after_pics/
The guy on Reddit claims the same amazing results in the past w every product listed. Read his posts from 1, 2 yrs ago. Etc. Everything is growing hair where he didn’t have it before. Careful
@Jake: thanks for the information. To be honest with hair regrowing products I believe the bad results more than the good ones.
Pyrilutamide Grey Market Stuff doesn’t seem to work because there are a lot of people with no effects on reddit.
People with hair loss normally have times with more hair loss and times with less hair loss.
If you ask 100 people and give them just water, there will always be people who will have regrowth or stopped hair loss and some people with worse hair loss. Only if there is a majority of them with clear results it’s a hint that the product is working.
Don’t look at individuals: Look at the majority with AGA.
Isn’t what you’re describing called a placebo controlled clinical trial?
If you ask 100 people and give them just water…and if you told them there could be side effects, some would get them, maybe even by just looking at the product.
The worst reaction I ever got from meds was from a steroid spray for severe hay fever of all things, but I do believe in the placebo affect in many cases. To elaborate on Yoda’s post: a mate of mine is a real wine snob and once declared he couldn’t “tolerate” red wine under $10 bc of his reflux. (God help us.) So one evening before a big dinner party, we switched around our go-to, bitter-as-hell $5 red and carefully poured it through a funnel into an empty and much more expensive wine bottle. We even used a wine aerator to make a show when pouring his drink. And you guessed it; throughout the evening he kept complimenting the wine – I kid you not! I love the dude, but what a goose. The placebo affect in all its glory. My gf of the time almost wet herself.
God knows what they sell to them on grey market.
Plus, every pharma company has the correct chemical formula and they doesn’t share every detail with the public or medical world what is the perfect chemical formula and how to dose it. Because everyone can copy their technology and put it under different name.
Small details in the formula makes big differences.
Kintor Pharma Announces Completion of Subject Enrollment in Phase III Clinical Trial of KX-826 for Treatment of Male Androgenetic Alopecia in China — Topline results not expected now until Q4 2023 – bummer.
Oh man! They were supposed to be done in Feb and then 1 month for data/results processing, so results any day now
This grew 7 more hairs per square centimeter than placebo. Placebo grew 15, Kintor grew 22. This makes no sense. Regardless, this is a garbage product. A full head of hair is 150-200 hairs per square centimeter. Doing nothing somehow grows 15 hairs per square centimeter. Using into will grow 22. Great. They’re going to have to twist and obfuscate the hell out of these results to get anyone to buy this crap.
BT,
Don’t be dense lad. Kintor understands well that pyri has subpar results in relation to finasteride. However their game plan since day 1 has been coadministration of GT and pyri . Those two therapies together will yield insane hair recovery with a growth stimi
Slightly off topic, but if >50 hairs/cm2 is barely noticeable (https://drserkanaygin.co.uk/hair-transplant-density-in-cm2/#:~:text=A%20full%2C%20healthy%20head%20of,to%20be%20noticeably%20thinning%20hair.)then theoretically won’t pyri combined with niostem be a functional cure for many?
There is no proof that Niostem works. They claim that even laser caps are much more effective than fin…
I am looking forward to GT20029.
True, it would be better if niostem published their data, but studies have shown LLLT to have comparable efficacy to fin (in fact I switched from min to LLLT recently after reading this paper – https://pubmed.ncbi.nlm.nih.gov/28396101/).
I’m also looking forward to GT20029, the more the merrier.
Kintor announcement today:
https://www.marketscreener.com/quote/stock/KINTOR-PHARMACEUTICAL-LIM-111325374/news/Kintor-Pharmaceutical-Pharma-Announces-Completion-of-Phase-I-Clinical-Trial-of-GT20029-in-the-US-43527282/
This hairloss cure isn’t doing much for the stock. The Kintor stock is down almost 60% for the year. I am now a believer in the products they have in the pipeline, but investors obviously don’t think it’s going to move earnings too much.
Hey does anyone know what vehicle is used for GT20029?
https://www.reddit.com/r/tressless/comments/14jcutw/pyrilutamide_six_months_update_new_progress_pics/
The account was suspended. It might have been a fake.
What am I missing? I don’t understand the excitement and it being touted as a cure. It’s around 10-15% of the growth per cm2 needed to get a full head of hair. Obviously, if you have 85% of your hair remaining, happy days, but a cure?
Who said it is a cure? Anything new that even maintains with no major side effects is a miracle. Minor additional growth is icing. It has been decades since Fin and Min were approved.
I’m not knocking progress, just saw some comments on Reddit touting it as a cure. Meant no offence. Was genuinely curious. I will refrain from commenting.
Did not take offense at all. If any cure is released, I will end this blog and you will know :-)
It’s also down to semantics. I class a ‘miracle’ as a cure or very close to one. As you know, I’ve shown CBD can do more than maintain with no side effects, but I definitely don’t call it a miracle. I assume no one’s interested in it because of the stigma and snake oil connotations. I wish it was a cure. Looking forward to that day. I like the idea this is topical though, which reduces chances of side effects and long term damage.
It could be another treatment to add to the stack. You could have a 5 alpha reductase inhibitor (finasteride), a growth stimulant (minoxidil), and an androgen receptor antagonist (pyrilutamide). Ru58841 is similar to this product but it isn’t fda approved. I think an fda approved drug that can prevent hair loss is a big win for the hair loss industry.
@admin, do you see much higher efficacy here than Breezula?
Have not checked and compared their original trial data recently, but I would assume similar.
What estimated date do you think the clinical trials of GT-20029 will end and its availability on the market? Before or later than 2026??
That’s strange. Maybe they discovered sideeffects? I see no other reason why they would do that.
I hope they learn and have a better trial design for GT20029 – I anticipate their results from trial 2 later this year. Should be superior to Pyrilutamide.
Hey admin when should we expect results of pyri phase 3? Thanks in advance.
I hope no later than next year.
I’d guess by the end of 2024. 52 week study however is ideal since the phase 2 study had gains by the placebo group. There may be no gains after 1 year and even more gains for the treatment group.
It will be the ultimate test. I’ve seen Breezula seem promising after 6 months but failed after 1 year. So we will see. I think if the drug fails though then GT20029 might not even happen because the company might be dead. They already have issues with other products and the stock isn’t performing well.
This is an email I got from kintor:
For the Phase III trial of Androgenetic alopecia(Male) in China, it is expected to finish by the end of 2023 and disclose the top-line data. If everything goes well, it will reach market late 2024 or early 2025.
For the Phase III trial of Androgenetic alopecia(Long-term safety) in China, we finished first patient enrollment on 19 July. A total of 270 male and female patients will be enrolled and the treatment period is 52 weeks.
Thoughts,admin?
It it reaches market before the end of 2025, I will take it. They have moved super fast so far. Late 2024 would be a real miracle.
Does that mean that it will release in these possible dates in USA too or just China?
A guy currently participating in the extended Phase III clinical trial of Pyrilutamide in China:
https://www.reddit.com/r/tressless/comments/17fx1z4/currently_participating_in_the_extended_phase_iii/
Another Trial … Kintor is not giving up.
https://www.marketscreener.com/quote/stock/KINTOR-PHARMACEUTICAL-LIM-111325374/news/Kintor-Pharmaceutical-Limited-Announces-That-the-Clinical-Trial-of-Its-In-House-Developed-and-Potent-46817606/