RIKEN is a Japanese government funded research institute, with one of their many areas of focus being hair loss cure research. They get very little in the way of public donations. This should change in FY 2021 due to their widely publicized request (which I discuss in the second half of this post).
RIKEN Updates
Update: October 12, 2021
Two interesting new developments this week in relation to RIKEN and hair made me update this post.
- First, they published some interesting new findings in relation to untangling the process of hair follicle development. Their scientists created a dynamic four-dimensional atlas that explains the origins and development of adult hair follicle stem cells. They call this a telescopic model and it analyzes the cellular dynamics and gene expression changes involved in hair follicle development. The actual study was published in “Nature” in June 2021 and is titled: “Tracing the origin of hair follicle stem cells.” Per lead researchers Hironobu Fujiwara and Ritsuko Morita:
“The findings could help drug developers to design new therapeutics to combat baldness and other types of hair loss.”
- Of more interest to this blog’s readers, a new lengthy summary of RIKEN and Dr. Takashi Tsuji’s work was just published in an online Chinese publication. There seems to be some kind of collaboration between RIKEN and ISEI Health in China’s regenerative medicine sector. Thanks to “Jan” for first posting the link. Key quotes from Dr. Tsuji below, including an implication that trials already started:
“The initial hair follicle regeneration clinical trial invested about 500 million yen. If the results are confirmed, I want to spend an additional 1 billion to 1.5 billion yen to gradually increase the number of trials.”
“Cost is about 50 million yen until the first 100 people. If it becomes 10,000, it will be reduced to 25 million yen, and after that, it will be reduced to 15 million yen. The more users there are, the more likely the cost is. reduce.”
February 11, 2021
I have never written a fundraising related post on this blog before. I have encouraged readers to donate small amounts in order to send people to major hair loss conferences twice. However, those fundraising goals amounted to just several thousand USD.
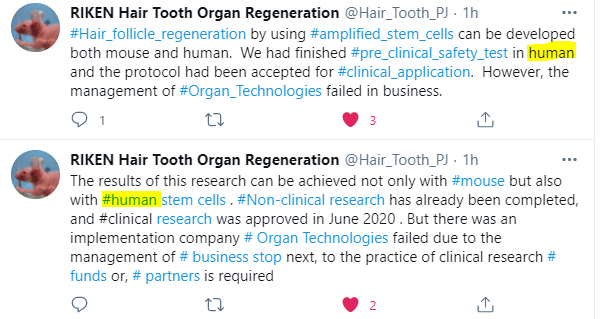
Several days ago, we learnt that Dr. Takashi Tsuji and his hair loss cure project was still progressing. Although he split from commercial partner Organ Technologies in 2020, his RIKEN (Rikagaku Kenkyūjo) team’s research continues to thrive. Next stop is clinical hair regeneration.
RIKEN Fundraising
However, the hair loss and teeth regeneration work from RIKEN now needs private funding.
“The team is seeking 500 million yen ($4.8 million) in donations from companies and individuals. The money will be used not only for clinical testing on the safety of the hair transplant technology, but also for other trials, such as regenerating teeth.”
- In FY 2018, RIKEN received only 37 donations for a total of approximately 8 million yen. Equivalent to $76,000 at current exchange rates.
- In FY 2019, RIKEN received 380 donations for a total of 25 million yen. Equivalent to just $240,000 at current exchange rates.
I wrote to the RIKEN team and suggested their using GoFundMe or contacting Allergan for investment. The latter has invested in 5 hair loss companies in recent years. In 2019, Allergan gave $25 million to Exicure, along with potential milestone payments of $265 million. RIKEN only needs $4.8 million.
Surprisingly, RIKEN replied to me right away. They were interested in my introducing them to the Allergan team, so I will need to figure that one out! More importantly, they said that they have created the below two links for those who want to donate:
Update: I have asked them to add a Paypal option. Hope they do so, as I prefer that option over paying by credit card. Someone on Twitter has also asked them to be more transparent by posting total funds raised to date information.
I later also asked RIKEN about approaching locally based Aderans. Their response was as follows:
“Aderans is a partner of hair diagnostic project but not hair follicle regeneration. I would like to meet a new partner of the hair regeneration for clinical application in human.”
Caveat
If you do donate to this cause, please note that the chances of any particular hair loss cure coming to realization are always abysmal. We have been disappointed over and over again for decades. I also doubt that private donations will get them more than $1 million, although I hope I am wrong.
I would suggest that the only reason to donate would be to support the overall goals of RIKEN when it comes to regenerative medicine. The implications of this work go across all of human biology. Not just hair and teeth.
Japan’s declining and aging population means that the country leads the world in anti-aging research. So these funds could indirectly benefit all of humanity. Clinical trials in Japan also proceed faster than anywhere else in the developed world. Especially when it comes to autologous regenerative medicine.
Of related interest, 10 of the 50 oldest living people in the world as of today are Japanese. That list is based on proven birth records. At the same time, Japan’s population has declined for 9 straight years, and a likely 10 straight years when new data is released.