Update: And in other important news from Japan from this week:
— Dr. Tsuji published a new paper several days ago.
— Pokemon Go was finally launched in Japan, its spiritual homeland.
Without any doubt, there has never been a better year than this one in the hair loss research world. The last four months have been especially fruitful, and I am not even considering a number of newer entrants in the field (just because most seem suspicious or are likely to produce best case results that will be akin to those from Rogaine). Key noteworthy recent developments:
- First, in April, Follica (which had been left for dead by many due to its numerous conflicting signals since inception in 2006) surprised us and is clearly alive and aims to release its product in 2018 in a best case scenario. The company’s majority owner PureTech stated in a summary document that “skin disruption alone was safe and generates new follicles as well as new hair“.
- Thereafter, in June, Aclaris Therapeutics’ CEO Mr. Neal Walker clearly stated at three difference investor presentation that “topical covalently bound highly selective JAK3 inhibitors” work in treating androgenetic alopecia and not just in treating alopecia areata. Alcaris will be conducting trials on this use of JAK inhibitors in 2017.
- Also in June, Histogen made a surprising announcement that it would start treating patients in Mexico in 2018. Like Follica, many people had left Histogen for dead due to the fact that the company has been involved in hair loss research for a similarly lengthy period of time with mixed signals in terms of efficacy, clinical trial and product release dates.
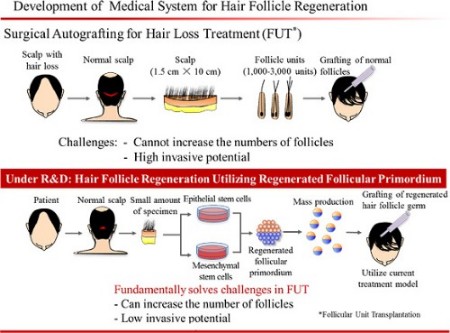
- Then, in July came perhaps the most exciting news ever in the hair loss cure research world. RIKEN/Dr. Takashi Tsuji, Kyocera and Organ Technologies have formed a partnership that aims to commercially release a product in 2020 that will essentially represent a cure for hair loss. 2020 is clearly a best case scenario assuming no major hiccups along the way. Mr. Tsuji is a world renowned and extremely well respected and modest scientist; RIKEN has access to Japanese government funds and to numerous leading scientists in the world of regenerative medicine; Kyocera is a private sector multinational behemoth with pre-existing hardware side technology and expertise that will likely be of much use to Dr. Tsuji.
- And finally, today the Shiseido/Replicel team finally announced the commencement of delayed clinical trials in Japan (see bottom part of this post).
RIKEN and Dr. Tsuji are conducting their research at the Kobe Biomedical Innovation Cluster (KBIC) in Japan in RIKEN’s Integrated Innovation Building.

Shiseido/Replicel Joins Kyocera/RIKEN/ Tsuji at the KBIC
Today, in yet one more major positive development this year, it was announced that the Shiseido/Replicel team has finally started its delayed 60-person (men and women included) Japanese clinical trials for its autologous cell therapy based RCH-01 product.

While the study will be conducted at two hospitals in Tokyo, the injected product(s) will be manufactured by Shiseido at their SPEC (Cell-Processing and Expansion Center) in KBIC. I discussed this facility in a post in 2014. It is located in the Business Support Center for Biomedical Research Activities (BMA) building in KBIC.
So Shiseido and RIKEN’s respective research and development work will be conducted at buildings within a mile of each other!!
I arrived at this conclusion after analyzing this map (both buildings are on the right side of the railroad).
While doing research on KBIC, I found that it is located on Port Island in the city of Kobe, and Port Island is an artificially constructed island. Would be quite something if humans end up creating new replacement hair follicles on a man-made island.