In 2021, the single most important drug in the fight against hair loss remains Dutasteride (brand name Avodart). However, it is not approved for that use by the US FDA. I have written numerous past posts on Dutasteride to treat male pattern hair loss.
The most useful one remains my Avodart reviews for hair loss post due to the vast number of reader comments. Also see my posts on Dutasteride injections (also knows as mesotherapy).
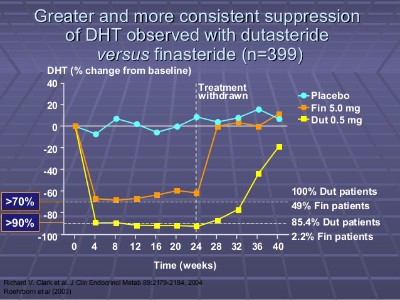
Dutasteride more Potent than Finasteride
Every week I search PubMed for the latest papers on Dutasteride and hair loss. This week, I discovered an important new June 2021 study that was just published. It is titled:
“Change in hair growth-related gene expression profile in human
isolated hair follicles induced by 5-alpha reductase inhibitors: dutasteride and finasteride.”
The findings of this study are quite interesting and related to something similar that I published in 2014 (see old post at bottom). Note that this latest work was led by Japanese researchers and funded by GlaxoSmithKline (GSK), the manufacturer of Avodart.
It is encouraging to see GSK still interested in Avodart for hair loss. They will almost certainly not try to get the drug approved to treat hair loss in the US or EU. To date, Japan and South Korea remain the only countries where you can officially use Dutasteride to treat hair loss.
In the rest of the world, the drug is prescribed to treat enlarged prostates (BPH) in older men. For hair loss, you need off-label prescriptions. Also note that on average, side effects from Dutasteride are worse than from Finasteride.
Although GSK is headquartered in the UK, this particular research was affiliated with the company’s Singapore subsidiary. Professor Manabu Ohyama (who I have covered in the past) gave advice on the study protocol and methodology.
Key Findings
The key findings of this latest paper on Dutasteride (and Finasteride) include:
- Dutasteride and Finasteride both suppressed the negative effects of testosterone on hair-related gene expression.
- In particular, the gene expression for growth factors FGF7, IGF1 and WNT5a.
- Dutasteride may have a stronger inhibitory potency to increase growth factor expression than Finasteride.
- Dutasteride’s greater efficacy on hair growth in comparison to Finasteride is possibly due to the inhibition of 5α-reductase Type 1; or due to greater inhibition of 5α-reductase Type 2 by Dutasteride when compared to Finasteride. My note: most likely both issues are at play per next conclusion.
- The authors claim that there results suggest that: “Type I 5 alpha reductase may play an important role in hair growth along with Type II 5 alpha reductase.
I forgot that this issue of the significance of Type 1 5α-reductase in relation to hair loss is still unresolved. My guess is that Type 1 is significant, but not quite as significant as Type 2. Dutasteride attacks both Type 1 and Type 2, while Finasteride only attacks the latter.
Make sure to also read my post on destroying androgen receptors on the scalp.
June 8, 2014
Dutasteride Works when Finasteride Fails
An interesting study by South Korean scientists was recently published in “The International Journal of Dermatology”. It measured the effect of Dutasteride on men with androgenetic alopecia unresponsive to treatment with Finasteride.
They took 35 balding people who did not see any improvement while on Finasteride 1 mg/day for 6 months. They then put those same people on Dutasteride 0.5 mg/day for 6 months. 31 of those patients completed the 6 months of treatment. Hopefully, the 4 who stopped treatment did not stop due to experiencing significant side effects.
Over three-quarters of these 31 patients saw at least some increase in hair count. Only 1 patient had a marked improvement, while 6 patients had a moderate improvement and 17 patients had a slight improvement. The remaining 7 patients did not see any improvement or worsening.
Side effects included transient sexual dysfunction in 6 patients (17.1%).