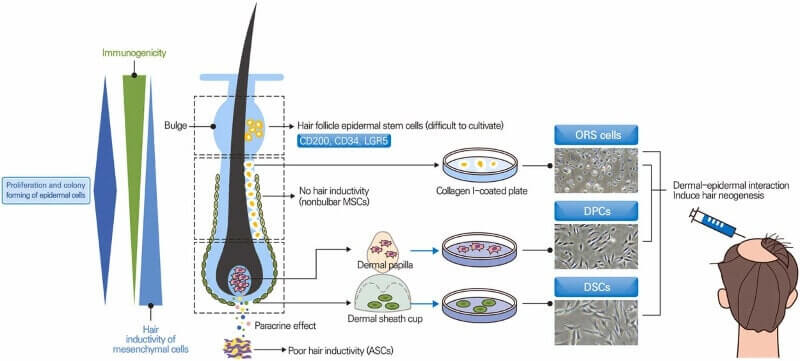
For those of us who have followed hair loss cure research over the past decade, the number one person of interest is Japan’s Dr. Takashi Tsuji. He previously aimed to release a hair loss cure by the end of 2020. This was prior to the onset of the global pandemic and his subsequent issues with funding.
Dr. Tsuji’s work occurs at RIKEN’s major research center in Kobe, Japan. Also of note, Japan is home to another groundbreaking hair loss researcher in Dr. Junji Fukuda (Yokohama University) and to Shiseido. The country is also where hair transplants were first ever performed and documented in the 1930s via Dr. Shoji Okuda (see “Okuda Papers“).
This post is very lengthy due to the fact that I cannot keep making new small posts each time there is an update by the Tsuji/RIKEN team. Instead I just add the latest developments on top. Make sure to also read my past writeups on Dr. Tsuji.
Update: November 27, 2022
Dr. Tsuji Now Aims for 2026
Reader “Theo” just sent me a link to an interesting recent interview with Dr. Tsuji in which he makes the following statement:
Now, we are drawing up a blueprint to assemble a new management team, raise funds, conduct clinical research, and start free medical care using “seeds” from fiscal 2026.
In 2021, the team’s venture company Organ Technologies (which was supposed to mass-produce the hair follicle “seeds”) failed to raise 2 billion yen in funding. It suspended operations, and later on Dr. Tsuji took over its debt in order to prevent patent loss.
Interestingly, Dr. Tsuji mentions that similar research is underway at Stanford University, Harvard University, and Yokohama National University. He suggests that all this competition will improve overall technology and reduce costs. This is the first time where I have seen him discuss any competition.
Update: A new April 2021 interview with Dr. Tsuji.
Update: They need $4.8 million in donations or funding in order to start clinical trials.
Dr. Tsuji and RIKEN Hair Multiplication 2021 Update
Riken has finally posted a major update on their site. You can translate from Japanese to English. Key sentence:
“We succeeded in developing a culture method that can amplify hair follicle epithelial stem cells while maintaining the ability to regenerate hair follicles.”
The official paper is published in Scientific Reports and titled “Expansion and characterization of epithelial stem cells with potential for cyclical hair regeneration”.
By using the culture method established in this study, the Tsuji team can create a large amount hair follicles from a small number of hair follicles. Moreover, these hairs will have normal regenerative capabilities and cyclical phases. The researchers were led by Dr. Takashi Tsuji and Dr. Makoto Takeo.
This update apparently represents a substantial improvement on their past work. The Riken team managed to multiply hair bulge-derived cells 4,000 times from one hair follicle. Dermal papilla cells were amplified in vitro about 100 times, so that from one hair follicle they could create 100 hair follicles. Key quote: “We have made great strides toward the realization of clinical application of hair follicle regeneration medicine.”
Note that this successful hair multiplication process entails extracting, multiplying and implanting hair follicles in mice. However, they also mention culturing human hair using specific proteins. Bulge-derived cells and follicles were amplified and about 80% regenerated 3 times or more in 80 days. In addition, the cultured cells had a periodic regeneration ability, just like normal hair.
I am disappointed that there is no news on when they will start their delayed human clinical trials. However, it has been a long time since we got such a lengthy update from the Riken team. Probably never this detailed.
Moreover, per another recent encouraging update, Dr. Tsuji is still keen on bringing this to market as soon as possible. Once he finds a new partner to replace Organ Technologies:
Clinical Trials and Practical use as Soon as Possible

On the official Twitter feed, they state that this represents successful cyclical regeneration of bioengineered hair. And the next stop is clinical hair regeneration. I prefer using the term hair multiplication, but probably not hair cloning.
Update: February 7, 2021
Thankfully, I was correct in my last prediction. Dr. Takashi Tsuji is still in action and rumors of his retirement were premature. A couple of weeks ago, an official RIKEN Twitter account started following me on Twitter. To be honest, the account started to look fraudulent and a bit unprofessional after each Tweet. I assumed it was a definite fake.
However, earlier today, I noticed this wonderful update on the RIKEN site:

So we are back in action, I am pretty sure :-) The Twitter account is official. Note that they will make a major announcement on February 10th, 2021 per the last Tweet:

Lets hope that Dr. Tsuji has found a new partner to replace Organ Technologies. Even better would be if he announces the beginning of human clinical trials or the results of completed Phase 1 trials.
Update: November 8, 2020
Rumors of Dr. Tsuji’s Failure Exaggerated
Recently, we learnt that Tsuji/RIKEN partner Organ Technologies had failed and was shutting down. A lot of people including myself were extremely disappointed. However, it seems like work at RIKEN continues. This month they published an interesting new study related to human skin.
More relevantly, in November 2020, Dr. Tsuji and his hair growth research was featured in a local NHK BS television broadcast. It was titled “Humanience-4 Billion Years of Plans.” Thanks to the various people who posted or e-mailed the above links. I sent the video link to “Fuji Maru” and hope he lets us know if it contains anything useful.
And today, to my utmost surprise, the most troll-like and negative commentator “jan” posted the most relevant link. Apparently, Dr. Tsuji and the RIKEN + Kyocera team’s patent was just updated. It is based on an April 2020 filing date, so probably just international approval formalities. Organ Technologies is still listed in there as a co-inventor.
Inventor(s):
TSUJI, Takashi; c/o RIKEN
TAKEO, Makoto; c/o RIKEN
OKAMOTO, Naokazu; c/o Organ Technologies
OGAWA, Miho; c/o Organ Technologies
MORI, Yutaka; c/o Kyocera
KISHIMOTO, Kyosuke; c/o Kyocera
Update: August 8, 2020

Youngjet added a summary video of his attendance of Dr. Tsuji’s Zoom lecture. On Twitter and Youtube, he told me that human clinical trials will start in 2020 after approval from the relevant Commission for Regenerative Medicine. To be honest, this is disappointing news as I was hoping human trials already started earlier this year.
Youngjet’s prior video at the bottom of this post and past statements from Dr. Tsuji both indicated faster progress. In any case, trials in Japan will not take as long as in the west, especially in the autologous regenerative medicine sector.
Update: August 6, 2020
Dr. Tsuji replied to my email, and it seems like no new announcements in the August 5 presentation:
Dear Admin,
Thank you for your interest in our research. There was no updates because the lecture was for graduate school students aiming to get fundamental knowledge. For further information, please refer to our laboratory website for the latest news on the progress of our research projects. Our laboratory is striving to advance research with the aim of fulfilling the expectations to improve quality of life for many individuals. We thank you for your patience and continued support of our research activities.
Sincerely,
Takashi Tsuji
Laboratory for Organ Regeneration
RIKEN Center for Developmental Biology Research (BDR)
Update: August 4, 2020
Dr. Takashi Tsuji Zoom Events in August
By now, most readers know that Dr. Takashi Tsuji is participating in several Zoom and web based online presentations in August 2020. I have sent a few messages to Fuji Maru and to ハゲリーマンちゃんねるin case they can attend any of these events. I will update this thread if they or others send me any new information.
Dr. Tsuji will be presenting via a Zoom conference on August 5, 2020:
- 11:30-12:30: “Organ regeneration by self-organization of stem cells.”
Then, on August 24 and August 25, Dr. Tsuji and RIKEN will be making a two-part web presentation.
- Part 1 = “Of hair science front line: from the basics of innovation to beauty and health.”
- Part 2 = “Overview of new products for FY2020.”

Update: June 26, 2020
New Interview
Recently, a number of readers have asked about Dr. Takashi Tsuji’s January 2020 article and interview in a Japanese magazine. Earlier today, reader “Lurker10” managed to purchase it and posted it on Imgur! Keep in mind that all the information in this article is from prior to the Covid-19 pandemic. So there could be some delays in the work’s progression. Update: It seems like the interview mostly covered Dr. Tsuji’s historical hair loss research related work per reader comments and translations.

Baldness will be Extinct
Update: August 14, 2019 — English version of below video is now published. Title: “Baldness will be Extinct! The Time has Come!!”
July 22, 2019
In June 2019, Dr. Tsuji gave some interesting quotes to the Financial Times about the future importance of Kobe when it comes to medical tourism. Also in June, Dr. Tsuji gave an important 4 page interview to Beyond Health.
Dr. Tsuji: Hair Loss Cure in 2020 or 2021
Recently, a Japanese hair enthusiast named “Youngjet” posted a video regarding his attendance of a recent lecture in Japan by Dr. Tsuji. Five people emailed me or commented on here about the below video!
After you translate the video, it seems like Dr. Tsuji will release his cure in 2020 and/or 2021. It will initially cost 20 million to 40 million Japanese Yen ($190,000 – $380,000 per today’s exchange rates). Prices will then slowly come down over the next decade.
Perhaps Dr. Tsuji gave us two numbers (20 million and 40 million) because some people will have one session and some will have two sessions?
Note that as of 2019, they are already testing this treatment in humans. Favorable Japanese regulations probably allow for this possibility, even though clinical trials are yet to be completed.
Make sure to see the results of my 2016 poll on how much you would pay for a hair loss cure. Only 12.5 percent of you voted for over $100,000!
— The guy (“Youngjet”) in the below video has the following Twitter account.
Perhaps the most important screenshot from the above video: