Update: March 21, 2024

CosmeRNA is now available in a lower-cost smaller container of just 1mL. It is priced at €84 (but currently has a 10% off sale offer) and will last for 1-2 months.
On Amazon UK, it is still also sold in the 6mL version for £310. It currently has an average rating of 3.5 out of 5 stars based on 27 reviews.
Recently, Bioneer’s CEO Han-Oh Park made a detailed video presentation in English at IMCAS 2024 that is worth watching.
The key active ingredient in CosmeRNA is “SAMI-RNA AR68″ (StearyldisulfidehexylDNA-2-PEG-45/SH-RNA-1). It is designed to selectively inhibit the expression of the androgen receptor (AR) in hair follicle cells.
Update: February 21, 2024
Make sure to check this pdf about CosmeRNA that was sent to me by Bioneer. The company has completed CosmeRNA’s Amazon Europe entry into Italy, Germany, France and Spain. Note that it previously only sold CosmeRNA on the Amazon UK website. In future, they will sell the product in Japan, Australia and Singapore.
Update: July 18, 2023
Bioneer has installed five additional high-capacity synthesizers in order to double the production capacity of patented raw materials for CosmeRNA, the world’s first RNA based hair loss relief cosmetic. Bioneer aims to secure more than 100 million of the world’s 2 billion male and female hair loss sufferers as CosmeRNA customers. Hopefully, the resulting economies of scale will allow a reduction in the product’s price.
Update: June 14, 2023
CosmeRNA is Now on Sale on Amazon
CosmeRNA is now available for sale via the UK Amazon site. It can be shipped and delivered to the US and other countries too. Make sure to review and rate the product if you use it for a few months.

South Korea just keeps on adding groundbreaking companies working on new hair loss treatments. The latest of these is Bioneer’s siRNA based cosmeceutical called CosmeRNA that targets the androgen receptor.
It can supposedly grow 1.3-1.9 hairs/cm per month, which is comparable to finasteride. Make sure to also read my post on how many hairs on a human head?
CosmeRNA was released in Western Europe in May 2023. Note that the top half of this post is all updates.
Update: May 2, 2023
- CosmeRNA online shop is now open! The price is €300 ($330) per bottle (vial), higher than originally expected. However, per the company’s site, it seems like the frequency of application will be reduced once all the androgen receptors are inhibited. Initially, the treatment is applied topically every two weeks for the first four months. Afterwards, once a month application may be sufficient to maintain the fuller hair.
Besides the key siRNA technology, the other ingredients are listed as follows:
Aqua, Phosphate Buffered Saline, Alcohol, Butylene Glycol, Betaine, Niacinamide, Stearyldisulfidehexyl DNA-2-PED-45/SH-RNA-1, Panthenol, Menthol, Sodium, Biotin, Citric Acid.
— It looks like Bioneer’s CosmeRNA Mall will finally open tomorrow. They describe the product as:
“The world’s first hair loss relief cosmetic based on RNA technology.”
CosmeRNA Finally Releasing in May 2023
Thanks to “Mathis” for the weekly updates. CosmeRNA’s slightly delayed release date is now confirmed for May 2023 per various news articles. Bioneer aims to get 100 million users within 5 years per CEO Park Han-oh. Release of the product in South Korea has been delayed due to some legal issues, although there is a lot of demand in the country.
The below YouTuber managed to contact a Bioneer employee and estimates the cost to be $67 per month. Based on a single $200 priced 6ml bottle lasting for three months, with 1ml recommended usage every two weeks. For comparison, the best laser hair growth devices cost $1000 or more, although the Lasercomb is much cheaper.
February 22, 2023
CosmeRNA for Sale at the end of March 2023
CosmeRNA related news just keeps coming and seems very encouraging. In the latest article from South Korean media, the company makes some bold claims:
- They expect sales of up to 160 billion Won (i.e., $123 million). This is quite extraordinary for a new cosmeceutical hair loss product that will only be sold in one region (Western Europe) for the time being.
- They state that “when the shopping mall opens at the end of next month, we will start full-fledged sales for European buyers.” So the product will be on sale by the end of March 2023.
- Since siRNA is mainly used for research, very few facilities have mass production capabilities. The scale of siRNA synthesis for most studies is at the mg level. Per this latest news, Bioneer can produce raw materials at a level of 1kg to 1.5kg per month,
- There are no major side effects and the product application does not leave any sticky residue.
Note that earlier this month, Bioneer announced that it will even sell on Amazon. And they will be partnering with Ace Biome for marketing and distribution purposes.
Registration on CPNP and SCPN Cosmetic Product Notification Portals
February 2, 2023 — Yet more encouraging news. Bioneer has also completed product registration with Britain’s submit a cosmetic product notification (SCPN) portal. Moreover, in January it participated in “IMCAS World Congress 2023” in France. this was in order to introduce Cosmerna to buyers around the world and to secure a distribution network.
December 26, 2022 — Bioneer announced that it has completed the registration of CosmeRNA on the cosmetic products notification portal (CPNP). The latter is a European cosmetics certification system and means that Bioneer can now distribute CosmeRNA throughout the EU.
November 23, 2022
CosmeRNA from Sirnagen (Bioneer) Releasing in 2023
This post covers a new topical hair loss product called CosmeRNA, whose website is now live. It is made by siRNAgen (South Korea), a subsidiary of Bioneer (South Korea). I briefly covered this company in the past, but now it deserves its own post.
The reason I am writing this post is due to a great new interview with siRNAgen CEO Dr. June Park. Both these new developments were sent to me by a reader who wishes to remain anonymous. Key quote from Dr. Park (also see her Linkedin):
“The first SAMiRNA product will be available for sale in the first half of 2023. We had an unconventional route to commercialization by developing a hair loss cosmeceutical product. CosmeRNA, named after cosmetic RNA, provided an early validation of our platform’s potential as a topical cosmetic product for androgenetic alopecia (hair loss).”
They will not need to conduct clinical trials since this is a cosmetic product! The actual release date is likely to be in early 2023 per another quote in the same interview. After first being done in South Korea, CosmeRNA’s safety study was repeated in Europe by Dermatest in 2022. Note that SAMiRNA is shelf stable in a solution for a year, so it can be applied topically.
Ms. Park anticipates the product launch to occur some time in early 2023. They will focus on the European market first due to the region’s more streamlined cosmeceutical trial and approval process. Via the Cosmetics Product Notification Portal (CPNP).
Note that I previously briefly covered siRNAgen Therapeutics in my post on OliX Pharmaceuticals. The latter is working on a hair loss cure involving RNA interference (RNAi) via asiRNA (asymmetric small interfering RNA). The aim is to reduce androgen receptor (AR) expression on the scalp.
SAMiRNA, Androgen Receptor and Hair Growth
Like OliX, siRNAgen is also working on reducing AR expression and re-growing hair. However, it is doing so via treatment with self-assembled micelle inhibitory RNA (SAMiRNA) nanoparticle-type siRNA. See their site for more on small interfering RNAs (siRNA).
They published an important study on this in Nature Journal in January 2022. The encouraging title of this paper is worth posting: “Weekly treatment with SAMiRNA targeting the androgen receptor ameliorates androgenetic alopecia.”
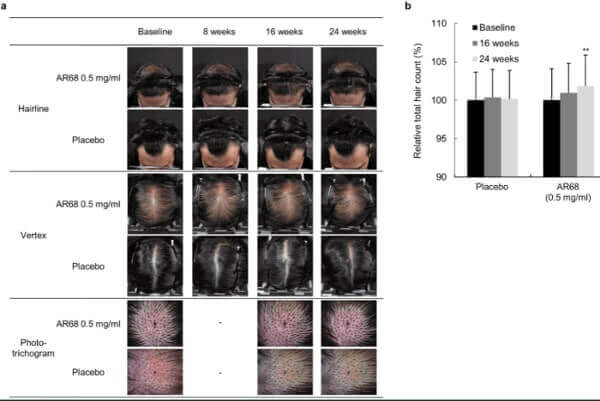
The product they use for androgenetic alopecia is a topical called AR68. It is classified as a cosmetic ingredient and will be called Cosmerna-68. In the above mentioned paper, they have before and after photos of a patient that I pasted below. The chart on the right shows the actual percent improvement with AR68 0.5mg/ml treatment versus placebo. They also state the following:
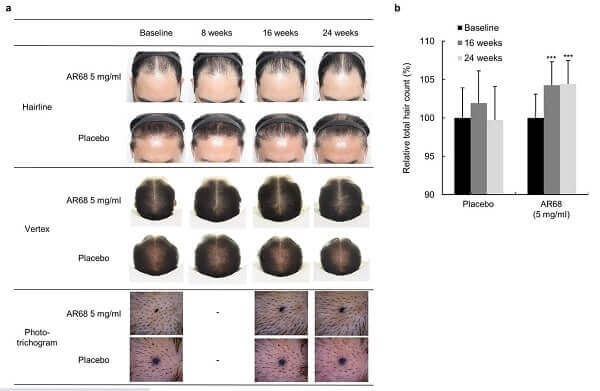
“In the low-dose (0.5 mg/ml) clinical study, AR68 was applied three times per week for 24 weeks, and through quantitative analysis using a phototrichogram, we confirmed increases in total hair counts. In the 24-week long high-dose (5 mg/ml) clinical study, AR68 showed average additional hair growth of 1.3-1.9 hairs/cm2 per month, which is comparable to finasteride. No side effects were observed. Therefore, SAMiRNA targeting AR mRNA is a potential novel topical treatment for AGA.”
0.5 mg/ml AR68 (3x a week) Before and After
5 mg/ml AR68 (1x a week) Before and After
The higher dose AR68 5mg/ml cohort’s before and after photos are here. The researchers claim that these results at 24 weeks are comparable to those from Finasteride. Safety was good in both low-dose and high-dose studies.
Summary
Is this a miracle? Most likely not for most severely balding people people. However, just as with finasteride, some people could see stellar results. And for those who only recently started to go bald, this could be a much needed non-DHT inhibiting product. With few if any side effects.
I am not a big believer in cosmetics, but this latest interview with siRNAgen CEO June Park is encouraging. She comes across as very intelligent and sincere. Note that in Bioneer’s September 2021 presentation, they describe CosmeRNA as a game changer hair loss treatment. It will have no side effect issues such as those seen with Finasteride (Propecia).


