In 2021, I wrote a post on microRNA (miRNA) and hair growth. In there, I discussed a new company working on messenger RNA (mRNA) based hair regeneration. And now we have RNA interference (RNAi) as a hair loss treatment, thanks to OliX Pharmaceuticals (South Korea) and its small interfering RNA (siRNA) based product.
Also not to forget, most recently, we have become excited by CosmeRNA, a SAMiRNA based hair loss treatment cosmeceutical that targets the androgen receptor. SAMiRNA is a new type of siRNA nanoparticle that does not result in innate immune stimulation. The number of different “RNA” acronym containing hair loss treatments in development has become sizable.
The bottom half of this post has a summary of OliX’s hair loss drug OLX104C (OLX72021) that targets the androgen receptor (AR). the top half is all newer updates.
Update: June 19, 2024
OliX Pharmaceuticals Completes Phase 1 Clinical Trials
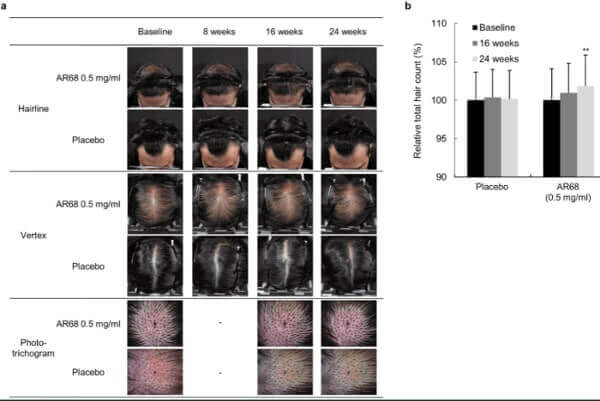
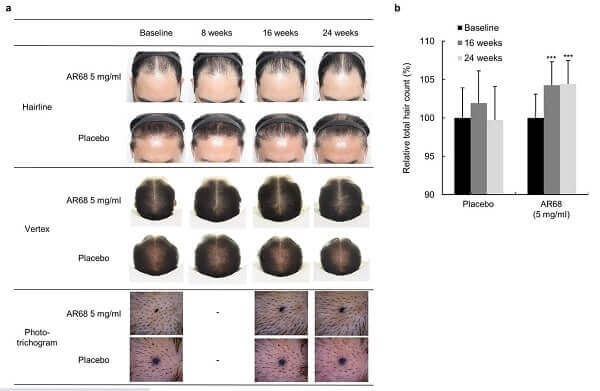
In a very surprising development, OliX Pharmaceuticals just announced that it completed its Phase 1 clinical trials in Australia ahead of schedule. Note that they call their hair loss treatment drug OLX104C, but call the “substance name” OLX72021.
The trial was conducted via a step-by-step increase in dosage. The drug was injecting intradermally into the hair loss patient’s crown region. Based on follow-up observations, safety was found to be excellent in three cohorts of treated volunteers. The company then decided to skip the fourth cohort. Official results are expected before the end of this year after 8 weeks of follow-up.
Meanwhile, apart from the injectable treatment, Olix is also developing a hair loss cosmeceutical and plans to launch it this year.
Update: April 12, 2024
At the just ended WCHR2024 conference, OliX Pharmaceutical made a presentation via Dr. Won Chong-hyun. This doctor is a dermatology professor at Asan Medical Center and is collaborating with OliX on the hair loss drug OLX104C (OLX72021) that targets the androgen receptor.
- In the news release on OliX’s own website, it says that OliX announced the “research results” of its hair loss treatment drug ‘OLX104C’ at WCHR 2024. Which would imply that the Phase 1 trials that started in June 2023 in Australia must have ended.
- Moreover, a Korebiomed article from today has the following quote:
“OliX Pharmaceuticals, a Korean biotech firm specializing in RNA interference technologies, recently announced the promising results of its hair loss treatment, OLX104C, at the 13th World Congress for Hair Research (WCHR 2024).”
Neither of the two news items discuss actual results. However, they must have been favorable enough for the South Korean company to attend and present at this conference in the US.
The presentation itself was titled: “Efficacy of Asymmetric siRNA Targeting Androgen Receptor for the Treatment of Androgenetic Alopecia.”
Update: June 13, 2023
Olix Pharmaceuticals Begins Clinical Trials in Australia
OliX Pharmaceuticals just announced that it has commenced Phase 1 clinical trials for OLX72021. This investigational RNAi therapeutic suppresses the hormonal activity that causes androgenic alopecia by reducing the expression of the androgen receptor (AR). OLX72021 is topically injected into the scalps of men with male pattern hair loss.
It seems like OLX104C has been renamed to OLX72021, although the former name is still the one listed on the company’s pipeline page. It could also end up being two separate products, with one being a drug and the other a cosmeceutical. I infer this from the earlier CEO update with quotes (see further below).
The 30 trial participants will be split into 5 cohorts that will get one intradermal injection of various doses (or placebo) and come back for follow-up after 8 weeks. The injection will be spread across 6 areas of alopecia near the crown region of the patients’ scalps.
Update: March 10, 2023
OliX just got approval from Australia’s Human Research Ethics Committees (HREC) to begin a phase 1 clinical trial of OLX72021. This candidate “inhibits the activity of hormones that cause male hair loss”. The effect lasted for more than three weeks in animal tests.
Update: In a March 23 article on this same development, CEO Dong Ki Lee states the following:
“We are also planning to launch hair loss cosmeceuticals in due time for the safety of OLX72021 to be confirmed in this clinical trial.”
“The company will develop OLX72021 not only as an RNAi technology-based new drug, but also as a cosmeceutical, which offers consumers accessibility and convenience of use.”
So far, we have only heard about Olix’s RNAi based candidate for the treatment of male pattern hair called OLX104C. It reduces the expression of the androgen receptor via small interfering RNA (siRNA). Perhaps the new product is just the old one renamed. I will update this post when I find out.
Asymmetric siRNA Targeting of the Androgen Receptors
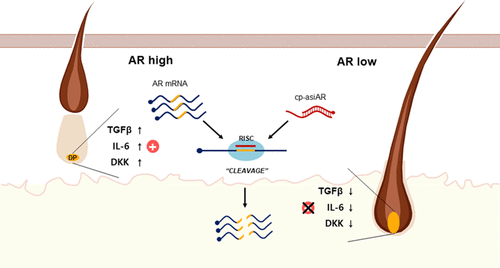
On November 22, 2022, OliX published preclinical research on its androgenetic alopecia treatment program, OLX104C. The results are very encouraging. Actual study is here and the researchers demonstrated:
“Efficacy of androgen receptor reduction, hair loss inhibition, and long duration of action in primary cultured human follicle dermal papilla cells (HFDPC) and rodent models of hair loss.”
They call this technology “cp-asiRNA targeting AR” and “AR-targeting asiRNA (cp-asiAR)”. The asiRNA stands for asymmetric small interfering RNA.
In May 2022, OliX raised $45 million and announce that its androgenetic alopecia product (OLX104C) will enter clinical trials later this year. Moreover, in August 2021, OliX obtained a Notice of Allowance from the US Patent and Trademark Office for OLX104C.
In June 2021, OliX signed an agreement with LGC Biosearch Technologies to accelerate production of asymmetric siRNA for the treatment of androgenic alopecia. Olix CEO Dong-ki Lee also presented at SMi’s 12th Annual RNA Therapeutics virtual conference.
February 10, 2021
OliX Pharma’s RNAi Hair Loss Product OLX104C
OliX Pharmaceuticals (South Korea) just made a major announcement. Their RNAi based hair loss product OLX104C successfully grew back hair in mice following just one single injection. The mice were suffering from androgenetic alopecia due to them being given excess dihydrotestosterone (DHT).
“We are advancing a novel and potentially durable approach to treating hair loss” — CEO Dong Ki Lee
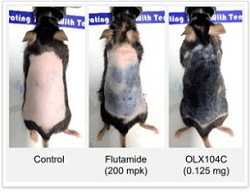
In this preclinical study, OLX104C was administered topically to a mouse model via an injection. The before and after photos are amazing. Interestingly, they compare the results to the anti-androgen Flutamide.
More importantly, OliX is the real deal. Their news page is very impressive with regular significant developments. This includes raising $37.2 million in December 2020. They also received US FDA Phase 2a approval in November, 2020 to treat hypertrophic scars.
siRNA
On OliX’s pipeline page, they state that small interfering RNA (siRNA) has limitations in terms of stability, delivery and toxicity. This is not a problem with RNAi therapeutics. Per their patent, they are using asymmetric siRNA to inhibit male pattern hair loss gene expression.
Hundreds of millions of people around the world took mRNA vaccines during the past few years. Even thought they did not go through rigorous clinical trials for five plus years. In effect, this will speed up scientific progress. Many people will no longer worry about long-term side effects for newer such RNA related drug candidates. Perhaps not a good idea, but I will not complain if it speeds up the realization of a hair loss cure.