I first wrote this post on Winlevi for acne in 2019 and often update it when updating my Breezula post. This is because both products are made by the same company (Cosmo Pharmaceuticals) and contain the same key key ingredient in clascoterone. Winlevi is much less potent than Breezula (1% versus 5%).
Winlevi clascoterone 1% cream is a first-in-class androgen receptor inhibitor that can be used by both males and females to treat acne. In was first approved by the US FDA in 2020. Thereafter, it was approved for sale in the EU and Canada in 2023.
Winlevi was originally manufactured by Cassiopea (Italy), prior to the latter’s 2021 takeover by Cosmo. More product details can be seen here. The Winlevi for acne reviews on WebMd and on drugs.com are not bad compared to other drugs.
Update: March 21, 2024
Winlevi is the #1 Most Prescribed Topical Acne Brand in the US
In Cosmo Pharmaceuticals’ latest news release, it makes the following statement:
“Winlevi continues to be the #1 prescribed branded topical acne product in the US.”
- More than 927,000 TRx (prescriptions) have been given out since launch.
- Winlevi has been prescribed by over 17,900 unique prescribers, representing over 90% of total healthcare practitioners in the US dermatology sector.
Also of interest, Comso has initiated new commercial partnerships that have made Winlevi available in the EU, UK, South Africa, Middle East, North Africa and South Korea. And most recently, in Australia via Sun Pharmaceutical Industries (India).
Update: November 1, 2021 — Sun Pharma announces that Winlevi Clascoterone 1% cream is now available for sale in the US. To be used for the treatment of acne vulgaris.
August 1, 2021
Winlevi, Cassiopea and Sun Pharma
Winlevi (clascoterone cream 1%) will now be released in the US market in the fourth quarter of 2021. Official website here.
On July 26, 2021, Cassiopea announced that it was partnering with Sun Pharma in order to commercialize and market Winlevi in the US and Canada. On July 29, 2021, the company also released its first half 2021 results.
The US FDA approved Winlevi® (clascoterone cream 1%) in August 2020 for the topical treatment of acne vulgaris. This prescription drug is a topical alternative to Accutane and Spironolactone. It is an anti-hormonal treatment for acne that targets the androgen receptor. It works by reducing sebum production and scalp inflammation.
Many people will inevitably try to use Winlevi on their scalps for hair growth (via off-label prescription). This is not recommended by the manufacturer.
August 27, 2020
Winlevi Approved by the FDA to Treat Acne

Cassiopea’s Winlevi topical androgen receptor inhibitor was just approved as an acne treatment by the US FDA. It is the first acne treatment with a new mechanism of action to be approved in 40 years.
The US FDA is also reviewing a new drug application (NDA) for clascoterone cream 1% to treat acne.
More information from Cosmo Pharma (Ireland — COPN:SWX), which owns 45 percent of Cassiopea (Italy — SKIN:SW).
CEO Diana Harbort quote:
“Dermatologists have said targeting androgen hormonal activity in the skin is ‘the holy grail’ of acne treatment for both males and females.”
August 23, 2019
I have covered Italian company Cassiopea numerous times on this blog. Primarily due to its Breezula topical product for male pattern hair loss. Earlier this year, Cassiopea announced very good 12-month Phase 2 clinical trial results for Breezula.
Clascoterone and Acne
Cassiopea is also working on an acne product called Winlevi. Both Breezula and Winlevi are based on the same key ingredient: Clascoterone. However, the dosage in the latter is significantly lower at 1%. This acne product is almost ready to come to market per Cassiopea’s product pipeline.
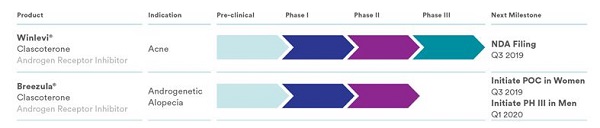
Earlier this week, Cassiopea made a major announcement. The company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking marketing approval for clascoterone cream 1% for the treatment of acne.
My Thoughts
Some interesting points in the press release and my thoughts:
- Clascoterone cream 1% targets androgen receptors at the site of application. This inhibits the local (skin) effects of dihydrotestosterone (DHT). Apparently, DHT is a key driver of acne lesion development. Most people wrongly assume it is solely testosterone.
- This acne treatment product will be safe to use in both males and females.
- If approved, clascoterone cream 1% will represent the first new mechanism of action in the treatment of acne in almost 40 years. Interestingly, if Breezula is approved for hair loss in 2021, it will be the first new topical or oral treatment for hair loss in almost 25 years.
Two Questions. Will it be possible to get Winlevi in higher doses in a Pharmacy? And where did you read Breezula release can be in 2021? Until now there was only info of 2022 release.
I said “approval” in 2021 rather than “release”. Maybe optimistic.
Yes, but, anyway, It is incredible that the last treatment approved for mpb was propecia ( a drug not designer to treat aga in first place ) in 1997 !
A poster on HLH called Desmond says there is an Australian compounding chemist willing to do this when winlevi is approved
surely if winlevi is approved, it will be much easier to approve breezula quickly?
I hope (but doubt) that is true.
I think that yes, specially cause you are the winlevi is approved for teens, that is a higher risk if it goes systemic at such young age. Well, after all, isotretinoin ( accutane ) also block DHT systemic. Hope FDA don’t blow it this time.
I might just go ahead and use the winlevi until breezula is relased
phase 3 in men for breezula has been delayed from q4 2019 to q1 2020
1 quarter delay in Hair Industry is nothing! :-)
And yes I think we can use several drugs and cosmetics for hair loss starting 2020 until we get better drugs. … I guess next year we start with Medipost/Celino, Polichem and Winlevi.
Phase 3 in men for breezula has been delayed from q4 2019 to q1 2020.
But now 2021…didn’t start phase 3.
Would there be a ANY benefit in using the 1% Winlevi on ones scalp for MPB? Or would the higher percentage be needed for it to be effective?
They said systemic penetration is the same in both candidates but it would not be effective to use Winlevi on your head because it is formulated to only penetrate facial skin while Breezula is designed to penetrate the scalp. Best to save your money and wait it out another year or so than waste your time applying something they already know doesn’t work.
I don’t know how well Winlevi will penetrate the scalp but the trial results indicate that the lowest percentage of Breezula tested (2.5%) was effective at maintenance +. So I certainly plan on giving Winlevi a try at the hairline to see what happens. It appears to be safe and as long as I can afford it, it’s an option.
Winlevi is 1% yes? That’s much weaker than Breezula (7.5%)** and in a formula that is not designed to penetrate the scalp. And you’ll need a dermatologist for the prescription. Too much for too little for me personally.
But I’ll definitely jump on this (Breezula) immediately when it is released (a report said 2022 but I’m sure that’s gonna be 2023). It looks to be a slight improvement over minoxidil, without the side effects…
…But it says: **”Phase 2 Study 34 men successful although results peak at 6 months and decline at 12 months”
So this is really just a short term topical.
I don’t know what to make of the declining results but I’m looking at this purely as a bridge to get to either Samumed or Follica. The 2.5% formulation was + 13 hairs so I don’t think it is unreasonable to give 1% Winlevi a try, even though my expectations won’t be high. I am also older and money isn’t a big factor for me personally……….understanding this isn’t an option for everyone. I am steadily and gradually moving backwards so this is really the only option I have left until approval of one of the above. Not sure what Yoda or MJones are thinking but their treatment history and current status seems very similar to mine.
Follica will be out first anyway, their phase 3 is only 3 months long I think
D1, The optimization studies were 3 months, (which is an excellent sign since it somewhat confirms that whatever the results, they can appear quickly), but I don’t think we can assume Phase 3 will be that short. I just looked again at some of the news releases and there is no mention of the size or length of the pivotal study. As of June 13, the optimization studies were continuing to enroll up to 60 men”
Follica’s pivotal study in androgenetic alopecia is expected to begin in 2019…
What evidence is there that Samumed will be better than Breezula?
Maybe Admin has more details but all I could find from Phase II was the term “statistically significant”. So if there is some concrete evidence, I am not aware.
Not sure what my opinion is worth but thanks for asking PQ, you’re a respected poster. My inclination would be to pass on Winlevi and wait for Breezula. I’d imagine this is “dose dependent” and 1% will be like rearranging the deck chairs on the Titanic. I’m already on an aggressive anti androgen regime but will seriously consider swapping out RU for Breezula when (if) it comes to market.
Thanks Yoda. Are you still maintaining? I could never get a comfort level with the quality level of RU or I would be on it too.
Yes PQ, I’m maintaining just fine,even some level of regrowth or thickening. My hair really took a dump after Dr. Lee went kaput. I tried various minox but nothing rivaled Xandrox. After a few years I jumped on Dut (was on fin 20 years) which stabilized the loss. RU really gave me a boost but I totally understand your or anyone’s reservations on using an unregulated Chinese chemical. For high strength minox I am using the Follics products, I believe these are pretty good. Just added low dose oral minox to the mix, I have borderline BP so killing 2 bird with one stone.
Thanks Yoda…………….where do you get RU……..Kane?
Yes, I buy the 5% premix from Anagen, Kane’s sister company. My math showed that mixing it myself wasn’t a huge cost savings…too much room for error.
Admin
You are much optimistic !!!
It is not a requirement if acne treatment is approved; this will be a reason for approving baldness treatment.
True Bekoo, but baldness treatment Phase II trail results were pretty good so reason for optimism! And both products inhibit DHT locally.
I believe this cream will work great for those with acne. I had really bad acne in my late teen years. Used over the counter nutrogena, proactive, also used benzyl peroxide prescription cream and antibiotics. None of them worked except for antibiotics but just marginally reduced it. My acne was cured by Propecia. After two months of use all my acne cleared up and I would get a zit here and there. My hair loss and acne cured within 2 months. I wish I had winlevi when I was 16 that would have saved me from acne for 3 years before getting on Propecia. I’m sure this will work off label for hair loss.
My point being dht definitely causes acne
Curious if you did accutane during that period.
i agree, however other hormones play a bigger role hence so many females having acne. females seem to continue having acne into their adult years where as males usually subside after puberty.
you are right though. every single friend i had with acne growing up has had problems with hair loss
Was wondering would a combo of Winlevi 1% +Retin A topical cream be as/near effective as the higher concentration Breezula?
why retin a?
Retin A helps the scalp to absorb the topical better.It was/has been used with Minoxidil.
Retinoic acid may also improve absorption of compounds because it speeds the shedding of dead skin cells, but current thought is that its synergism with minoxidil is due to the upregulation of the sulfotransferase enzyme that transforms topical minoxidil, which is a prodrug, into its biologically active form.
Some sulfotransferases are involved in hormone sulfation, but I think clascoterone acts immediately on hormone receptors. Not sure.
Transdermal penetration is tricky.
tretinoin (retin a) will help with cellular turnover also.
this is just as important as minoxidil and can be used on its own.
the usual prescribed amount with formulated minox is 0.025% i always found that too strong so i use half of that.
retinol, however at a high amount 0.5% is a great way to get your skin ready for retin a. it will turn into retin a within the skin,
i actually think the retinol 0.5% was just as effective, if not even more so. its been 7 years since using it but it did wonders.
Hey Admin,
Curious your take on Trinov now that it’s been out for a while.
What’s the community consensus seem to be?
Thanks for another great article!
That’s great news, but as I understand it, standard approval time after submitting the NDA is 10 to 12 months. If so, we have about a year to go for Winlevi.
Good news, it means that the bases on which breezula rests are safe for health
But maybe another 2/3 years for FDA approval for Breezula for me
I kept having this suspicion that my severe acne during my teen years and taking Accutane somehow were related to my hair loss in my adult life
Body builders report bad sides with accutane.
I wonder if this treatment can also reduce sebum production because of oily skin type.
My parents wouldn’t let me take Accutane when I was 16. They knew about the sides and even my doctor said Accutane has bad sides, including hair loss and depression. So I took the benylperoxide and antibiotic root. Accutane is some serious stuff. Its crazy how Propecia just cleared my acne up so quickly. My skin was super oily, zits, some cystic acne and 2 months later clear skin and hair loss halted. I was excellent responder. I hope something new comes soon where I can respond to excellently again. Rogaine isn’t cutting it now.
@MJones: Did your sebum level go down as well? Also, did you have oily and acne prone scalp? Did that respond favorably to propecia?
Ignore my earlier comment. I didn’t see this.
Follicum approved patent in Korea:
Follicum AB (”Follicum”) today announces that the Korean patent office (”KIPO”) has approved the company’s patent application for stimulation of hair growth.
With the approval in Korea, patent number 10-1992282, Follicum has a patent protection in Korean up to and including year 2032. The patent protects the company’s peptides in general and for stimulation of hair growth in particular.
CEO Jan Alenfall comments: “This patent approval is exciting as Korea is an important market for hair growth products. Other than the formal approval from Korea this patent has been approved in the US, Europe, China, Australia, Russia, Hong Kong and Japan. We now have a very good protection regarding our candidate molecule FOL-005 and other peptides on significant markets around the world.”
…Steady rollin’…
Is it possible to purchase breezula in europe before being approved by FDA?
My sebum remained oily though especially on my scalp and it still does. Not drenching oil. I need to wash my hair everyday because I’m diffuse and hair gets clumpy if I don’t. My acne went away regardless of the sebum. So to answer your question, the days I don’t work out my sebum levels are normal and minimal except in humid Philly weather in summer. Winter time my scalp is dryer and less oil. Before Propecia face was super oily especially teen years. I had thick dense hair so didn’t notice oily scalp. Once my hair started shedding at 19 I noticed oily scalp as I lost density. Face very oily too. Hopped on Propecia acne gone , mpb stopped bit still oily if I don’t shower
Mjones at age 60:
https://www.theguardian.com/commentisfree/2019/aug/24/is-simon-cowell-new-face-vision-of-deepfake-future
Why you people still have hope on stuff like Breezula… it’s nothing if not less than another Dr. Brotzu lotion…
At best a new cure will enter the market only in 5 or 10 years.
If we didn’t have hope Lorence we’d be like you, a bitter, jaded, negative person. What’s your point dude? If Breezula comes to market it has zero parallel to Trinov. It’s a drug/medical treatment, not a cosmetic.
The CEO says that the phase III trials for breezula are only 6 months long. Why are they not bothered about doing it for a year?
Great news, I guess we could use the 1% version to maintain + small regrowth. Also, surely there’s a way to increase absorption (without dermarolling) such as applying after warm shower etc.
Admin I dont get the link ?? Me at 60? Simon Cowell lol. I’ll take it as a compliment since he is rich and gets all hot women.
I wanted to post a reply with that link showing current cosmetic possibilities, and you are a good person to reply to when needed :-)
Strange but OK admin lol
Purely an FYI, representing nothing new re the pivotal trial but Puretech’s Follica pipeline page has changed to now include 2 product candidates. FOL-004 and FOL-005. 005 is for skin rejuvenation. You can click on each candidate for a bit more info………..mainly projected market size, with alopecia dwarfing skin rejuvenation. I note that the 005 “key differentiation” is the same as for 004 as it refers to follicle and not skin creation, so perhaps website changes are still under development.
Sweet
We can dodge bull dog face and get our hair back at the same time
I’ll takeit
Egghead approves
Just colour to go now!
Replicel has updated his website
Replicel press release. Check it:
https://www.replicel.com/news/replicel-life-sciences-retains-dermatology-sales-and-marketing-executive-to-guide-the-global-commercial-strategy-for-its-automated-dermal-injector
Isnt this just for skin though?
It’s for their stupid dermal injector. Don’t get your hopes up. They’ll use this for other cosmetic purposes. Don’t see anything about their use for hair loss or anything about sisheido. These companies need to release something or go away. Sht is getting old…..really old
I think they said that it was their injector and its consumables that were soon coming to market.
I wonder when RCH-01 releases, be it from Shiseido or Replicel, will Tsuji consider them a competitor? Because what they do is not direct cloning but I’m just wondering if both the procedures being out around the same time will make them drive down each other’s prices.
Their injector will be used for the treatment, is an essential part of the project. Despite this, reading their presentation, I don’t perceive a company with clear ideas. Which seems to be Puretech(owner of Follica).
Replicel … a bad joke …
Admin, why do you post comments from posters that have zero to add? See the “bad joke” guy, he never says anything of value, just pissing and moaning. Once in a while is understood as we all can get impatient but has this guy ever said anything constructive or informative? With all due respect, overall I applaud your low level of censorship even though you’ve silenced me a time or two.
@Yoda
Apparently baldness is not your only problem …
Sincerely
The bad joke guy
If Replicel are a joke, why did Shiseido invest millions in completing their joke? Why did Shiseido not buy into Follica or Aclaris or Samumed? Hell with their money they could have bought them all and owned the market.
Or maybe the board member who decided to invest in Replicel is soon to be fired. Doubts grow with their ever increasing delay of the results.
More positivity from the happy, positive winner! :-)
Larry, do you ever add anything to the conversation besides negative quips? No posts on your experience with treatments, info you’ve found, research, or opinions grounded with some sort of basis. Your posts only seem to be that everything is a scam, sucks. Whaaaa-whaaa, little boo boo Lorence, life is soooo unfair!!
https://www.thesun.co.uk/news/9699106/baldness-cure-stem-cell-treatment-reverse-hair-loss/amp/
Has anyone of you seen this before?!
Fake/ scam
Hi John
Can you please elaborate? How do you know?
– The before and after pic can be achieved with a different combing.
– The Indian doctor in Bangalore who invented this “cure” in 2014 is not on the Forbes rich list.
– The testimonials on the company’s websites don’t mention this product and have consistent grammatical errors indicative of each being written by the same person.
– Their cases studies web page shows no pictures.
– Their chat responses to my question (which they refused to answer) were automated and meticulously scripted.
First of all thank you scot for the reply, I have to sent a question about their certifications and haven’t yet received an answer.
With that said there are a lot of clinics using that treatment of regenera activa
And isn’t it an Spanish invention?
Anyways thank you again
Hey Scott, good question. Well follica is funded by Puretech so sisheudo couldn’t touch it. Samumed is funded by some big politicians and other important people behind the scenes so they want that money themselves. Follicum doesn’t work only on leg hairs lol. I agree they do see potential in sisheudo. If they can get it to work they’ll make bank. I’m just tired of all delays, repeat phase 1s and no update in ages. Follica will be out soon and they will reap the benefits. Easier to get, administer and no need to fly to Japan and be on a waiting list to get injected by replicel. Plus I heard Japan will be only offering it to local Japanese citizens until final phase 3 is completed.
@MJones, market mechanisms will dictate otherwise, if it proves that RCH-01 is effective. I believe the American expression is Money Talks. Perhaps if the treatment relied on a special limited substance, but this is not the case.
For people that are willing to visit this forum everyday and try untested chemical compounds, without proof of safety or effectiveness, on the hearsay of someone they have never met, you would not think that a couple of trips to Japan to get a REAL cure would be so inconvenient for them. Assuming of course it will work.
Japan is after all, a very developed country. Nobody is asking you to visit Bosnia, or Uganda or anywhere like that.
@Mjones Follica out soon? When is soon? 2021?
Soon in his dreams
Admin, I’m still wondering if you’ll be covering the latest Replicel update or if it’s not that important?
Am I right in thinking that we were supposed to get some Tsuji results in July? I’m sure someone (Greg?) said something like that.
@Paul One of the lead scientists on the Shiseido team for RCH-01 said that the results for the phase II trials would be open in July but we haven’t heard from them yet.
Thanks, Greg.
Replicel isn’t going to initiate the next hair trial until they have commercialized their dermal injector which was going to be released in Europe in 2016—then in 2016 it was going to be tested in 2017 and released in 2018—then in 2018 it was 2019—now it’s 2019 and it might be released in Hong Kong and Europe in early 2020—AND in the May 2019 Company presentation it says it will be released in 2023! (Seriously) All for a dermal injector that was developed in 2013.
Now we’re talking RCH-01 First-in-Japan launch in 2022 after saying it was a ‘potential near-term market launch’ since late 2016 —And now the May company presentation says by the end of 2020 there will by ‘new clinical data announced from the RCH-01 clinical research in Japan (financed by Shiseido)’ —By the end of 2020 for data—
So here’s the punchline: Replicel said in 2011 that they would have efficacy data in 2012 and “that means that a treatment in the U.S. would probably be available in the next five years”
*crowd laughs*
And we still don’t know if it works!
*crowd laughs so hard their eyes fill with tears*
You guys better pray follica comes out soon because nothing else will that will give 30 to 100 cm2 growth. SM, CB will be the new Propecia miniox combo for 2020s. Same growth and maintenance results just newer generation that will help out new blading guys. It will work but won’t be any better than what we have now. Maybe less sides. This is what I assume so dint start bashing me haha. The time we get our hands on tsuji will be 10 years out unless you got money to stay in Japan long term and people on this instead to get it during a long wait list. I’m doubtful on sisheido, gut telling me they will postpone and do another phase 2.
Admin- any word on that HairStim topical?
Shiseido can safely be written off. Japanese somehow managed to get WORSE results than RepliCel’s phase 1.
That thing is def getting shelved.
Mjones if Follica can fix or dramatically improved my Norwood 5 I will be very happy and I will forget about hair cloning for a while, maybe even years.
Follica and some sort of topical anti-androgen are all that anyone should really be waiting for tbh. They are the only things that are going to be both affordable and actually work.
PureTech’s annual report or whatever came out yesterday and Follica is still doing their phase 3 this year. Their end-of-phase II meeting with the FDA went well and the results they got after just one passage of the device and chemicals absolutely produced worthwhile.
But it says on Puretech’s site that Follica is a “series” of in-office visits. I would guess that the average person needs about 3 or 4 hits of Follica to get strong density based on the results they’ve already released.
Basically if you pair that with Breezula or some other topical AA, that’s the cure. People can complain and whine about still having to a daily regimen, but that’s their problem.
We’re in the final steps where there will be a superior option to a transplant and an AA which you don’t have to worry so much about side effects and worst of all, the effect it might have on your children and it will all cost less than a transplant. It’s absolutely worth it.
I really hope Follica can figure out a way to boosts the 30cm2 to 100cm2. Not sure what they will be doing in phase 3 is the effectiveness tests over? Or will they continue to make the product better? Sorry if that’s a stupid question lol.
They’ve stated before that they will continue to look to improve the treatment even after its on the market.
But again, to get up to 100cm2 you can just go until you get it.
that guy, ok thanks
I always thought that follicles you have to do for your own at home every day?!
Is follica a treatment which you get in a clinic? It would be much better for me to go to follica 5 times and pay 5 times then doing this every day at home like minoxidil
I remember reading on there website a while ago like 2 years ago that they said it would be “at home” but then now I read on there website and also by news articles that this device is going to be only available if you go a to a clinic and done by a professional who is trained. That’s fine with me I’d rather have someone do it for me so I can get good results.
Me too. It is much better a procedure in clinic then doing it at home
Tsuji news where you at
I keep my hair really short. I get acne on my scalp. So…
how come oral jak inhibitors didn’t work for AGA? but topical did? doesn’t make sense.
This is solid news. However I do get nervous seeing that cassiopeia stated 9% of adolescents will have problems with the HPA axis and that 5% of adults will as well. Being I’m an antenna for side effects, this makes me nervous.
Tomjones. Can you provide a reference for the problem with HPA axis? Or explain it in a little more detail. Like you, I’m an antenna for side effects.
Search winlevi side effects and you will get the insert outlining side effects. This was a major disappointment to see.
It would be great if we knew what month in 2021!!
Early 2021 for a drug approved in late August is a long rollout. I’ve seen drugs hit the pharma shelves in < 3 mos. after approval. But at least it's something to finally look forward to in 2021. Not sure how much of an effect that low dose will have on hairloss. But Breezula has a high probability of approval now.
This would take a long time but what if you could use Follica’s device on your donor area a while after you get a transplant when everything heals and then you use Follica’s device on the scar line to create new DHT resistant hairs and then you do a transplant again it would unlimited DHT resistant hair you just keep repeating the process.
Mjones have you tried exosomes? I’m considering it but I’m not really a fan of the injections because I had PRP done in 2016 and it hurt so bad and I don’t think it really did much but then again I didn’t get it with Acell maybe that would have helped idk
Hi woofy. No prp and exosome is all a scam in my opinion. Its expensive for the minor or no results given. I would just go for a fue for 5k you will spend in exosome. Prp may work for other types of hair loss but not aga. It’s a great therapy for sports injuries
So…is this suppose to regrow dormant hair or just protect what you currently have?
Primarily the latter.
This are simple amazing good news !!!!! It is the first topical antiandrogen approved ever !!!!!!!!!!!!!!!!
WAY-316606 and Sandalore
the product is planned to launch in the Italian market by the end of the 2020
Does that WAY and Sandalore grow thick terminal hair? Or is it another bs Brotzu lotion?
YES, WAY and Sandalore grow thick terminal hair
NO WAY
Mjones This seems to work https://scholar.google.com/scholar?hl=en&as_sdt=0%2C10&q=Diclofenac+gel+male+pattern+baldness+&oq=gel#d=gs_qabs&u=%23p%3DGARSJgUJnm4J
Woofy what do You thjink about latest Tsuji updates? it is not optimistic imo
Is this competition for Follica?
https://www.beautiphi.co.nz/cosmetic-medicine-men/hair-loss-protocols/
Take a look at the list of growth factors etcetera.
Great find Netshed. New Zealand and Australia both have some unique and interesting hair growth products, companies and researchers.
i agree, c-pharmacy has to be the worlds pioneer in non surgical hair restoration. and their prices definitely show that they know it.
incredibly smart man doing the work over there. he was actually the one who challanged the “adequate” number level of vitamin d in australia and he won.
Just looked at the before after pics of it netshed. Doesn’t look good imho. Nothing to be excited about.
Hi Admin! I was looking to buy Winlevi (Clascoterone) online and I couldn’t find anything. Any news about the release?
You need a prescription.
Is there any way to import winveli in Europe from US or Canada? Other anti-hormonal treatments available in Europe are only for women.
FYI I asked my doctor about Winlevi for hair and he gave me a prescription (which I can’t fill until it is released) but he said he wasn’t sure how much effect it would have given the strength of only 1%. But he said he didn’t see any downside assuming no listed side effects. I am going to apply at the hair line only, but over apply, and then see what happens. I don’t have anything else to try at the moment. And who knows, maybe only 1 or 2 % will have a better effect when used in combination with Dut and Minox than just Winlevi alone.
Thanks for the update!
Keep us updated. Curious to see if it works. You’re right though, it may be too weak (may need to wait for the stronger stuff).
I’ll let everyone know if I think it is having any effect but given I probably won’t start for 3-4 months depending on actual availability, plus, since it will basically be a maintenance only product (absent a big unexpected surprise), we are probably talking about next spring before I could give much of an assessment.
if anyone had been following the comments in the last thread and has any questions or seeking advice you can email me at, quentintaramino@yandex.com
Disclaimer: this is in no way affiliated with hairlosscure2020, this is my own personal experiences and depending on response may be a longer waiting time.
i am checking with the therapeutic goods association here in australia what i can legally do.
thankyou
Hi Bro ,
Can this specification below be useful to me?
Amount Per Serving: % Daily Value*
Calories 17
Total Carbohydrates 2 g <1%
Protein 2 g 4%
Sodium 20 mg <1%
*Percent Daily Values (DV) are based on a 2,000 calorie diet.
Typical Amino Acid Profile (Milligrams Per Serving)
Essential Amino Acids (EAAs)
Tryptophan 36
Valine 115
Threonine 132
Isoleucine 124
Leucine 211
Lysine 181
Phenylalanine 65
Methionine 39
Conditionally Essential Amino Acids (CEAAs)
Arginine 52
Cysteine 50
Tyrosine 61
Histidine 38
Proline 136
Glutamic Acid/Glutamine 302
Nonessential Amino Acids (NAAs)
Aspartic Acid 202
Serine 97
Glycine 64
Alanine 95
it would be better than nothing imho, though still not enough,
that looks similar to the aminos found in collagen.
if it had said 8grams protein, and contained vitamin c/zinc then yes.
be careful of tryptophan, especially if you are taking any other sort of medication.
ive seen people with serotonin syndrome and they were literally gray skinned, and lifeless..
Methionine by itself can be as effective as finasteride to some people.
that is probably the most important thing for mpb.
arginine, lysine and cysteine being the best for regrowth.
has anyone heard of a compound called rhodanide?
it was originally used on sheep to thicken their furr and give farmers a bigger yeild.
only a few companies use or know of this and have it in their products.
from my experience, it slows shedding but also increases hair growth rate excessively.
ever had a shed from minoxidil and then it takes months to see those new hairs coming in?
i do think rhodanide helps speed up the regrowth of stronger hairs.
retin a also.
i dont know how, but i was going grey. and im pretty sure that it is the amino acids that have changed that, but i look at my hair now and i see one grey hair? it is incredible what some things.. that are currently on the market and that are legal can do..
Feel like I should have just asked you to write part of a post on amino acids (if you wanted) as your comments seem very useful. It’s a shame they might get dispersed across posts.
I can do that, not sure how great my grammar is though.
No worries. Will figure out something!
I e-mailed you FYI.
Just too bad Methionine probably significantly decreases lifespan in animals (you can google it – lots of info).
“probably”
Unfortunately there’s a lot of studies on it just from the last 10 years – especially on the fact that it counters the beneficial effects of calorie restriction (google: methionine lifespan). Just a short citation from one 2019 study: “Methionine restriction (MR) is one of only a few dietary manipulations known to robustly extend healthspan in mammals”. I’m not an expert on the subject though.
Hell if it reduces sebum its a win. My scalp gets so oily in the areas of hair loss.
Great point. Plus reduce the inflammation and itching.
I would be willing to try this solution for hair but I’m not sure how easy it will be to apply the acne preparation on the scalp. Preferable solution would be to make a very simple DIY compound (or obviously the 7.5% solution from Cassiopea, which I doubt we will ever see at this point given that we are now 2 years delayed…). Hopefully once Winlevi is officially released and on the market we can locate a much, much easier to locate and cheaper supply of CB-03-01 with the additional benefit of additional safety information etc. I would be willing to try the 1% version on my hairline but I can’t see this being practical to use for the full scalp.
When breezula’s phase 3 begin?
Phase 3 in men for breezula has been delayed from q4 2019 to q1 2020.
But now August 2021.
Their CEO: “Until the end of 2021, our priorities are supporting Sun Pharma in the successful launch of Winlevi® and the continuing the development of clascoterone solution for AGA, an area that has not seen innovation in 20-30 years.”
So hopefully soon..
My guess is that Breezula is as good as gone. All we need is a reliable online CB-03-01 supplier/compounder and we will know quickly if this is a suitable treatment.
Guess it goes to show that my above comment was without merit. Latest news from Cassiopeia indicates that they are still working on clascoterone for AGA:
“During the first half of 2021, activities were focused on the preparations for the US commercial launch of Winlevi® (clascoterone cream 1 %) and advancing the development of clascoterone solution for androgenetic alopecia (AGA).
The Phase II trial investigating clascoterone solution for the treatment of androgenetic alopecia (AGA) in females was completed in the reporting period. Top line results will be available in 3Q 2021.
Progress was made in the development of a new Patient Reported Outcome (PRO) Questionnaire for AGA which has been requested by the FDA to be used in the future Phase III trials of clascoterone solution for AGA in males.”
I also saw in the press release about their deal with Sun Pharma that “Following this transaction, Cassiopea will be expecting substantial revenue streams for the foreseeable future and will be well funded to continue the development of its innovative dermatology pipeline.” I take this to mean that they now have the funding to advance Breezula.
One Woman’s Hormonal Acne Journey with Winlevi:
https://www.allure.com/story/winlevi-hormonal-acne-experience
I have always had many acne problems, and the onset of my androgenetic alopecia at the age of 18 corresponded with a massive secretion of sebum on the scalp. It has been shown that DHT is found in very abundant quantities in the sebum of the skin. Now I am 28 years old and my skin’s sebum secretion has normalized, although I still have oily skin but to a lesser extent. I have tried all possible remedies against acne throughout my life, topical, oral and none have ever been effective, my skin on my face is full of scars, so an effective medication against acne for people who suffer from it aggressively. Now I’m very happy, and also alopecia and acne have a common origin: hormones! However, my uncle, may he rest in peace, died with all his hair at the age of 70 and his face full of scars from acne, therefore we must be aware that a medication like Kintor Pharma, for example, is effective against acne. and alopecia, it will never be able to prevent hair loss in those of us who have it written in our genetics, like all other medications.