There are 50 locations across 4 countries (US, Georgia, Germany and Poland) for Breezula Phase 3 Trials. If you are in the US, please search for your state (or nearby state) in both the SCALP1 and SCALP2 breakouts on this page.
Breezula Phase 3 Clinical Trials
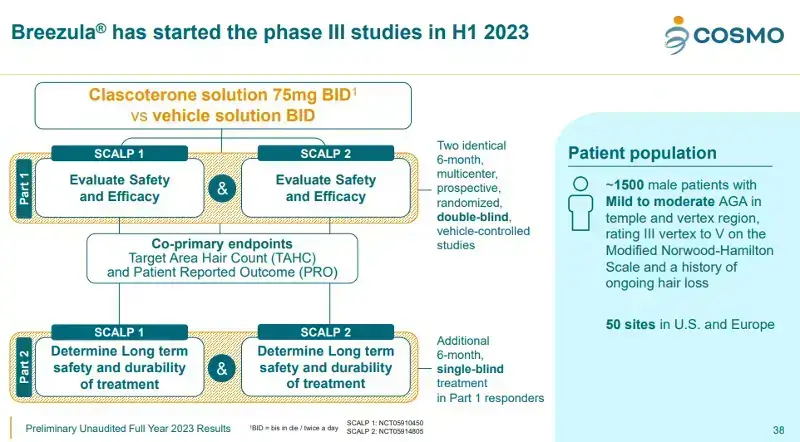
In my recent post about Cosmo Pharmaceuticals’ update on Breezula’s Phase 3 clinical trials, we learnt that the enrollment is still less than 60% compelete. This despite the fact that these trials started in June 2023. As of March 2024:
- 348 out of a planned 726 patients have been recruited in study CB-03-01/37.
- 507 out of a planned 726 patients have been recruited in study CB-03-01/38.
One reason for this slow pace of recruitment is that the inclusion and exclusion criteria are quite stringent.
- While both the clinical trial page links below say that only men above 18 are allowed, our own “Yoda” tried to get in and was told that men abve 55 are also not allowed.
- Only men who have mild to moderate androgenetic alopecia (AGA) in the temple and vertex regions (ranging from III to V on the Norwood-Hamilton Scale) are eligible. They must also have ongoing hair loss.
- You have to revisit the center several times over 6 months, and apply the topical clascoterone solution on a daily basis.
- You need to have not taken finasteride or dutasteride within 6 months of visit 2. And not taken topical minoxidil within 12 weeks of visit 2. They need to be able to figure out only Breezula hair growth results via its androgen receptor (AR) antagonist mechanism of action. This requirement may dissuade a majority of hair loss sufferers from participating.
There are a total of 8 inclusion criteria and 14 exclusion criteria that you must read before deciding to volunteer.
Another major issue is that there are various Breezula clinical trial URLs floating around, and past company announcements were a bit confusing about locations. Moreover, even in the below two links, the location section is a scroll-through box where you can only see one location on the screen at a time.
So I am pasting all the 50 locations (across 4 countries) below in the hopes that I can help at least slightly speed up recruitement.
SCALP 1 Locations
United States
Arkansas Locations
North Little Rock, Arkansas, United States, 72116
The Petrus Center for Aesthetic Surgery and Hair Transplantation
Contact: Gary Petrus, MD
Contact: Terri Kim Rogers
+1 501-614-3052 Kim@DrPetrus.com
California Locations
San Diego, California, United States, 92123
Therapeutics Clinical Research
Contact: Neal Bhatia, MD
Contact: Hector A Bailon
+1 858-571-6800 hbailon@therapeuticsresearch.com
Florida Locations
Jacksonville, Florida, United States, 32224
Mayo Clinic, Department of Dermatology
Contact: Alison Bruce, MD
Contact: Matthew Dwarika
+1 904-953-3551 Dwarika.Matthew@mayo.edu
Miami, Florida, United States, 33125
University of Miami
Contact: Mariya Miteva, MD
Contact: Olumide Ojoola, MD
+1 305-243-8205 oxo160@med.miami.edu
Louisiana Locations
Baton Rouge, Louisiana, United States, 70809
DelRicht Research
Contact: Ira Thorla, MD
+1 225-412-0316 info@delricht.com
New Orleans, Louisiana, United States, 70115
DelRicht Research
Contact: Sarah Jackson, MD
+1 504-336-2667 info@delricht.com
Michigan Locations
Detroit, Michigan, United States, 48202
Henry Ford Health
Contact: Linda Stein Gold, MD
Contact: Fanar Razoky
+1 248-219-4304 frazoky1@hfhs.org
Minnesota Locations
Minneapolis, Minnesota, United States, 55455
University of Minnesota
Contact: Maria Hordinsky, MD
Contact: Jaime Nugent
+1 612-625-8625 speck007@umn.edu
Nevada Locations
Las Vegas, Nevada, United States, 89148
JDR Dermatology Research
Contact: James Del Rosso, MD
Contact: Melissa Jacobson
+1 702-964-2425 mj.drdr@gmail.com
New Jersey Locations
Berlin, New Jersey, United States, 08009
Hassman Research Institute
Contact: Michael Hassman, MD
Michael.Hassman@cenexel.com
Contact: Mark Capichana
+1 856-753-7335 m.capichana@cenexel.com
New York Locations
New York, New York, United States, 10022
Diane S. Berson MD, PLLC
Contact: Diane S Berson, MD
Contact: Kiera Murphy
+1 212-355-3511 staff@dianebersonmd.com
Stony Brook, New York, United States, 11790
Derm Research Center of New York, Inc.
Contact: Elyse Rafal, MD
Contact: Dawn D’Angelo
+1 631-880-9660 ddangelo@drcny.com
North Carolina Locations
Charlotte, North Carolina, United States, 28277
On Site Clinical Solutions
Contact: Catherine Pointon, MD
Contact: Alexandra Perez
+1 704-995-1198 aperez@onsiteclinical.com
Texas Locations
Austin, Texas, United States, 78759
DermResearch
Contact: Janet DuBois, MD
Contact: Hadie Mendoza
+1 512-349-9889 hadie@vialtrials.com
Frisco, Texas, United States, 75034
DelRicht Research
Contact: Brent Spencer, MD
+1 927-807-5787 info@delricht.com
San Antonio, Texas, United States, 78213
Progressive Clinical Research
Contact: Mark Lee, MD
Contact: Marta Reyna
+1 210-614-5557 mreyna@progclin.com
Georgia (the country)
Batumi, Georgia, 6000
LTD Health
Contact: Lela Beridze, MD
Contact:
Natia Bezhanidze
+995 597 51 47 34 natiabezhanidze3@gmail.com
Tbilisi, Georgia, 0114
JSC Curatio
Contact: Maia Datuashvili, MD
Contact: Neli Bakuradze
+995 599 90 23 54 Nelly_bakuradze@yahoo.com
Tbilisi, Georgia, 0159
Kanveni National Center of Dermatology and Venerology
Contact: George Galdava, MD
Contact: Victoria Sulava
+995 593 324 578 sulavav@yahoo.com
Tbilisi, Georgia, 0159
Tbilisi Cancer Center
Contact: Lally Mekokishvili, MD
Contact: Nino Kapanadze
+995 577 79 78 06 Ninucakap@yahoo.com
Tbilisi, Georgia, 0160
Aversi Clinic
Contact: Sergo Dadashyan, MD
Contact: Maka Katsia
+995 593 34 32 66 mkatsia@yahoo.com
Tbilisi, Georgia, 0160
Medi Club
Contact: Nino Lortkipanidze, MD
Contact: Mariam Kutidze
+995 595 80 19 89 m.kutidze@mcg.ge
Tbilisi, Georgia, 0179
David Abuladze Georgian-Italian Clinic
Contact: Josephe Kobakhidze, MD
Contact: Tinatin Shaishmelashvili
+995 558 50 75 55 tinatin.shaishmelashvili@gmail.com
Tbilisi, Georgia, 0186
LTD “Medinvestment”
Contact: Irakli Dartsmelia, MD
Contact: Tamar Loseliani
+995 577 73 63 33 tata.ioseliani@gmail.com
SCALP 2 Locations
United States
Arizona Locations
Scottsdale, Arizona, United States, 85255
Investigate MD
Contact: Brenda LaTowsky, MD
+1 480-440-5985 research@investigatemd.com
Florida Locations
Hollywood, Florida, United States, 33021
Physician’s Institute of Cosmetic and Reconstructive Surgery
Contact: Jonathan Weiser, MD
Contact: Wanda Bargot
+1 945-964-4113 wandabargot@yahoo.com
Georgia Locations
Atlanta, Georgia, United States, 30329
DelRicht Research
Contact: Robert Springer, MD
+1 770-417-4454 info@delricht.com
Michigan Locations
Troy, Michigan, United States, 48084
Revival Research Institute
Contact: Ali Moiin, MD
Contact: Sikar Grewal
+1 248-564-1485 grewal@rev-research.com
Warren, Michigan, United States, 48088
Grekin Skin Institute
Contact: Steven Grekin, MD
Contact: Claudia Attala
+1 586-759-5525 claudia.attala@adcsclinics.com
Missouri Locations
Wildwood, Missouri, United States, 63040
DelRicht Research
Contact: Christopher Kling, MD
+1 504-336-2667 info@delricht.com
North Carolina Locations
Huntersville, North Carolina, United States, 28078
Piedmont Plastic Surgery and Dermatology
Contact: Nicole Seminara, MD
Contact: Jeffrey Decker
+1 980-900-9229 Jdecker@onsiteclinical.com
South Carolina Locations
Spartanburg, South Carolina, United States, 29307
Advanced Dermatology & Cosmetic Surgery
Contact: Amylynne Frankel, MD
Contact: Ashley Davis
+1 864-574-0017 ashley.davis@adcsclinics.com
Tennessee Locations
Thompson’s Station, Tennessee, United States, 37179
DelRicht Research
Contact: Pezhman Shoureshi, MD
+1 629-895-1775 info@delricht.com
Texas Locations
Pflugerville, Texas, United States, 78660
Austin Institute for Clinical Research
Contact: Edward Lain, MD
Contact: Xiomara Duarte
+1 512-279-2545 XDuarte@atxresearch.com
Sugar Land, Texas, United States, 77479
Acclaim Dermatology LLC
Contact: Syed Ali, MD
Contact: Carina Espinoza
+1 832-770-6388 carinae@acclaimderm.com
Washington Locations
Spokane, Washington, United States, 99202
Premier Clinical Research
Contact: William P Werschler, MD
Contact: Celeste Gray
+1 509-505-1815 CGray@PremierClinicalResearch.com
Germany
Berlin, Germany, 10117
Clinical Research Center for Hair and Skin Science, Dept. of Dermatology, Venereology and Allergology, Charité – Universitätsmedizin Berlin
Contact: Ulrike Blume-Peytavi, MD
Contact: Annette Andruck
+49 30 450 518 458 annette.andruck@charite.de
Berlin, Germany, 10629
Emovis GmbH
Contact: Guido Burbach, MD
Contact: Julia Welle
+49 (0)30 439741220 julia.welle@emovis.de
Düsseldorf, Germany, 40212
Privatpraxis Dr. Hilton & Partner
Contact: Rodrigo da Mota
Contact: Melanie Momm
+49 (0) 211 86 29 28 90 momm@dr-hilton.de
Freiburg, Germany, 79098
Dermaticum-Privatpraxis für Dermatologie
Contact: Rolf Hoffmann, MD
Contact: Danuta Sobczak
+49 (0) 1702838441 dsobczak@t-online.de
Hamburg, Germany, 20095
Eurofins | bioskin
Contact: Walter Wigger-Alberti, MD
Contact: Sabine Schendel
+49 40 606 89 743 Sabine.Schendel@cpt.eurofinseu.com
Mainz, Germany, 55131
Department of Dermatology and Allergy, Johannes Gutenberg-Universität KöR
Contact: Petra Staubach, MD
Contact: Evelyn Mueller
+49 (0) 6131-17-2944 evelyn.mueller@unimedizin-mainz.de
Poland
Katowice, Poland, 40-081
Centrum Medyczne Pratia
Contact: Kamila Płaczek, MD
Contact: Paulina Majka
+48 721 201 421 paulina.majka@pratia.com
Katowice, Poland, 40-611
Centrum Medyczne Angelius Provita
Contact: Magdalena Kolanko, MD
Contact: Agata Puchowicz
+48 721 210 110 a.puchowicz@angelius.org
Krakow, Poland, 30-033
Centrum Medyczne All-med Badania Kliniczne
Contact: Grażyna Pulka, MD
Contact: Alicja Wolska-Król
+48 12 422 34 271 allmedpl@gmail.com
Kraków, Poland, 31-011
Centrum Nowoczesnych Terapii Dobry Lekarz
Contact: Malgorzata Dyczek, MD
Contact: Katarzyna Gajda
+48 690 000 367 katarzyna.gajda@dobrylekarz.com.pl
Kraków, Poland, 31-559
Diamond Clinic Sp. z o.o.
Contact: Barbara Rewerska, MD
Contact: Aleksandra Kubajewska
+48 571 241 412 a.kubajewska@diamondclinic.eu
Lublin, Poland, 20-412
Pro Life Medica
Contact: Agnieszka Sajdak-Wojtaluk, MD
Contact: Agnieszka Saja-Górecka
+48 537 827 727 a.saja-gorecka@etg-network.com
Warszawa, Poland, 02-637
Narodowy Instytut Geriatrii, Reumatologii iRehabilitacji im. prof. dr hab. med. Eleonory Reicher
Contact: Mariusz Sikora, MD
Contact: Marta Kurek
+48 603 315 033 marta.kurek@spartanska.pl
Warszawa, Poland, 02-661
Carpe Diem Centrum Medycyny Estetycznej
Contact: Weronika Mołga, MD
Contact: Paulina Litkowska
+48 608 888 888 “paulina.litkowska@gmail.com
Łódź, Poland, 90-436
Dermoklinika Centrum Medyczne s.c. M. Kierstan, J.Narbutt, A. Lesiak
Contact: Aleksandra Lesiak, MD
Contact: Małgorzata Szulc
+48 690 056 595 badaniakliniczne@dermoklinika.pl


