Update: February 2022
Samumed Ends Hair Loss Program
The biggest disgrace in the history of the hair loss world just happened. At least on par with the past failures of Histogen, Aderans and Intercytex. Update 2023: And now Follica too.
Biospice (formerly Samumed) just layed off its staff and axed its baldness program. After fooling us for a decade and implying that FDA approval was close. Their insane valuation was always a red flag. But I still got carried away after they finished Phase 3 clinical trials in 2021.

In a surprising twist, Samumed just rebranded itself as Biosplice Therapeutics (San Diego). CEO Osman Kibar has stepped down. The new CEO is Cevdet Samikoglu. In 2021, the company is supposed to release much anticipated results of Phase 3 clinical trials for its hair loss product.
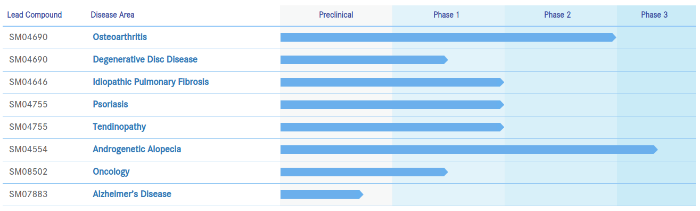
Biosplice Therapeutics (formerly Samumed)
Biosplice also just raised $150 million in new equity financing investment. This latest round of financing will allow the company to accelerate the development and launch of its osteoarthritis drug candidate Lorecivivint.
The name Biosplice (rather than Samumed) more closely resembles the company’s alternative pre-mRNA splicing technology. It seems like splicing has becoming the company’s new buzzword or brand. Rather than the prior Wnt signaling pathway. From their updated site:
“Biosplice’s mission is to restore health by delivering first-in-class therapies that harness alternative splicing.”
Biosplice’s small molecule technology will make use of alternative splicing to target the CLK/DYRK family of kinases. Oncology and cancer treatment (Cirtuvivint) will be a key focus area of research. Alzheimer’s disease and other degenerative condition will also remain key target areas of treatment.
The renamed company already has a short wikipedia entry.
Dalosirvat (formerly SM04554)
I was initially concerned at the rebranding and the new company’s focus shifting away from the Wnt pathway. However, then I noticed that their androgenetic alopecia drug has been renamed from SM04554 to Dalosirvat.
In my opinion, this is good news. If their soon-to-release Phase 3 clinical trial results are expected to be poor, why go through a product renaming?
Update: The guys on HLT seem skeptical. Some think that the renamed company might be preparing itself for an initial public offering (IPO).
Regarding Dalosirvat, the redone website says:
“The ongoing phase 2/3 study is fully enrolled with data availability expected in 2021.”
More information on the Wnt pathway activator Dalosirvat hair growth molecule can be found here.