Note that prior to its hair multiplication work, Shiseido was already well known in Asia via its Shiseido Adenovital Adenosine based products.
Update: February 8, 2021
RepliCel just came out with a new detailed corporate update. It warns Shiseido to adhere to their mutual agreement. i.e., deliver the full recently completed Phase 2 clinical study data set of RCH-01 in Japan. Shiseido calls the same product S-DSC (dermal sheath cup cells).
Interestingly, Replicel is in the near future also planning to start its own Phase 2 clinical trials for the hair growth product (outside of Asia, where Shiseido has the rights). Probably due to Replicel’s recent success in getting new funding via a partnership with MainPointe Pharmaceuticals.
Update: October 14, 2020
Some rare good news this year. Shiseido just started its second multi-center clinical study on the efficacy and safety of extensive repeated injections of cultured human autologous hair follicle dermal sheath cup cells. The treatment will be tested on 36 male and female pattern hair loss patients. Interestingly, they do not mention Replicel’s name anywhere, indicating continuing unresolved partnership issues.
However, Replicel’s latest Tweet (h/t “Paul”) does not hold back:
Shiseido has quietly launched its second multi-center clinical study of RepliCel’s RCH-01 for the treatment of hair loss in Japan.
Shiseido Clinical Trial Update
Update: March 27, 2020
Shiseido’s latest trial results are finally getting global publicity. Yesterday, lead researcher Dr. Ryoji Tsuboi was interviewed by the Asahi Shimbun. The title of the article clearly states that this technology entails transplantation of ones own cells. Not surprisingly, The Daily Mail has even more details.
Shiseido’s method involves autologous transplantation of dermal sheath cup cells. I am very optimistic that Japan’s favorable regulations will lead to this technology reaching the market in the next several years.
Dr. Tsuboi:
“The result of our study was very encouraging, We were able to show that the study could help develop a new treatment for hair loss.”
Update: March 6, 2020
Update: Fuji Maru sent me the following Japanese press release from Shiseido. The company plans to conduct further clinical trials that will administer DSC cells in multiple areas of the scalp.
Autologous Cell-Based Therapy Success
Reader “John Doe” just discovered this new study from Shiseido. As always, I am assuming that Shiseido uses both Replicel’s technology as well as its own-in house technology. Lead author is Dr. Ryoji Tsuboi from Tokyo Medical University. Toho University Ohashi Medical Center was also involved in the trial.
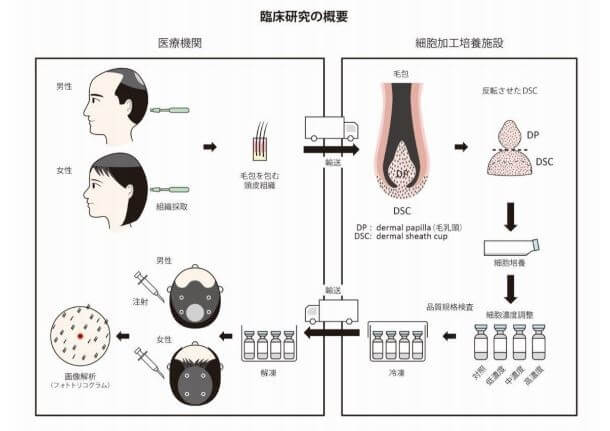
The treatment entails autologous cell–based therapy using dermal sheath cup (DSC) cells, which surround the dermal papilla (DP). Cell manufacturing was conducted at the Shiseido Cell-Processing and Expansion Center (SPEC) and Incubation Center. Both male pattern hair loss and female pattern hair loss patients were treated. 50 males and 15 females, all between the ages of 33-64.
The positive: Total hair density and cumulative hair diameter at injection site significantly increased at 6-months and at 9-months. They seem to imply that this was true for all patients, but maybe someone can access the full study and let us know. Side effects were limited to none in all participating volunteers.
The negative (possibly): The “positive effect was temporary until 9 months”. This statement does not make linguistical sense, and is likely a translation issue. Are they actually trying to say that hair growth was temporary for all of the first 9 months after injection?
Update: Thanks to reader “Left4Bald” for sending me the whole study. They state that the best results were at 6- and 9-months post injection. 12 months had “reduced hair growth”, which is still unclear wording. Further data in the full study indicates that there was a very slight average decline in hair count from 9 to 12 months. Interestingly, older men and women saw best results per phototrichogram before and after images.
Also of significance, 3.0×105 DSC cells was the lowest dose tested. It still elicited a significant increase in total hair density and cumulative hair diameter compared with the placebo. Higher doses did not translate to superior hair growth. The authors suggest that this could be due to an overload of cell debris and immune reactions. In effect causing a poorer environment for the remaining viable DSC cells.
In any case, we should prepare for an eventuality where we need once-a-year DSC injections. Hopefully, Shiseido succeeds in rapidly commercializing this autologous hair growth treatment in regulation friendly Japan.
Update: November 26, 2019
Shiseido Japanese Patent Published
Someone named “Paul” (thanks!) just posted a link to Shiseido’s official Japanese patent, which was released in Japan in May 2019. Paul found this link on an investing forum, and he also posted a bunch of information about stock prices. I deleted the latter, as I do not want to encourage blog readers to buy or sell Shiseido or Replicel stock.
Ultimately, whether “Shiseido decides to do so OR not to do so” will decide everything. The patent indicates optimism about the technology. However, nothing is set in stone for sure. The English translation of the patent is extremely informative if you expand each section and wait for it to load. Or you can open the whole Japanese pdf too and use translation software.
The big question I have is what portion of the technology comes from Replicel (licensing), versus from Shiseido in-house?
The inventors are listed as:
- Tsutomu Soma
- Jiro Kishimoto
- Sayaka Koide
- Hitoshi Okochi
- Masahiro Kiso
Update: November 26, 2019
Replicel Year-End 2019 Update
RepliCel released its 2019 year-end update today. It includes discussion about its partnership with Shiseido. Regarding Replicel’s cellular therapy (RCH-01) for androgenetic alopecia, the key points are that:
- Shiseido’s clinical study in Japan is now complete.
- Shiseido will soon announce on whether it will commercially launch the product in Japan or conduct further clinical testing.
- Replicel is holding off on Shiseido’s final decision before pursuing any further Phase 2 trials. The former’s Phase 1 trials in Europe were successful.
- The Replicel and Shiseido dispute is still unresolved.
It seems like Shiseido could still launch the product in Japan soon. No doubt aided by the country’s new faster regulations for marketing when it comes to cellular therapy.
Replicel’s proprietary injection device, RCI-02, is on schedule to get approval and be used in much of the developed world in 2020.
Replicel-Shiseido Partnership
March 23, 2019
I used to cover Replicel (Canada) and its Japanese cosmetics behemoth partner Shiseido at least a few times per year until last year. At some point, I got skeptical about the RCH-01 autologous cell therapy hair loss product being released any time in the near future.
![]() Besides typical delays, Replicel and Shiseido had some unclear conflicts regarding their partnership.
Besides typical delays, Replicel and Shiseido had some unclear conflicts regarding their partnership.
However, Replicel published two important updates this month, the second of which is of most interest to us:
Per the first link above, the disagreement regarding the agreement between Shiseido and Replicel remains unresolved. However, it is not subject to any litigation or arbitration at this time.
The key money quote is from the second link:
“While the Company’s RCH-01 product for hair loss due to androgenic alopecia may be launched in Japan much earlier if Shiseido decides to do so, current planning anticipates the potential for all four products to be on the market in Japan by 2022.”
The implication seems to be that:
- Shiseido could release the product well before 2022. See my past post on the company’s new research facility in Kobe, Japan.
- Even if Shiseido does not release the product earlier, it will quite possibly get released in 2022 in Japan by Replicel.
See my past post on Japan’s new laws fast-tracking stem cell therapies and clinical trials.
Another key quote:
“Unlike anywhere else in the world, one well-designed cell therapy trial in Japan, approved by their regulatory authorities, has the potential to lead to product market launch.”
Replicel is clearly focusing on a “First-in-Japan” strategy due the country’s favorable regulatory environment. Shiseido has rights to the Asian market when it comes to RCH-01. However, it seems like Replicel can still release the product in Japan in 2022 if Shiseido does not do so?
Hard to make this conclusion for certain without knowing much more about the two companies’ legal contract. In any case, this is a great development.
Further references:
- A discussion of Replicel’s technology in two videos.