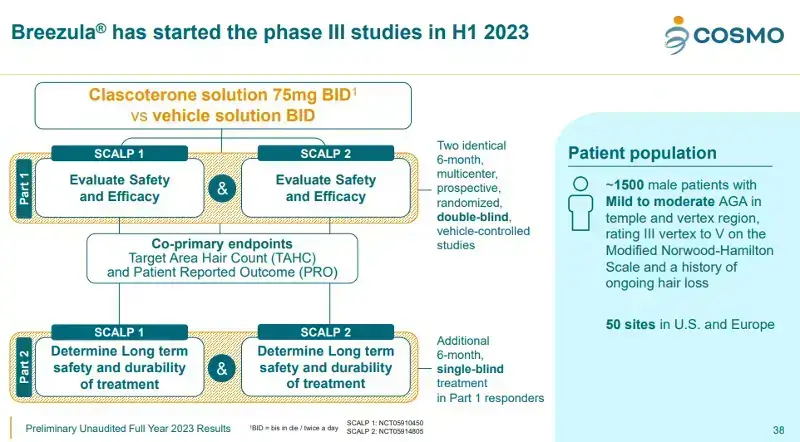
There are 50 locations across 4 countries (US, Georgia, Germany and Poland) for Breezula Phase 3 Trials. If you are in the US, please search for your state (or nearby state) in both the SCALP1 and SCALP2 breakouts on this page.
Breezula Phase 3 Clinical Trials
In my recent post about Cosmo Pharmaceuticals’ update on Breezula’s Phase 3 clinical trials, we learnt that the enrollment is still less than 60% compelete. This despite the fact that these trials started in June 2023. As of March 2024:
- 348 out of a planned 726 patients have been recruited in study CB-03-01/37.
- 507 out of a planned 726 patients have been recruited in study CB-03-01/38.
One reason for this slow pace of recruitment is that the inclusion and exclusion criteria are quite stringent.
- While both the clinical trial page links below say that only men above 18 are allowed, our own “Yoda” tried to get in and was told that men abve 55 are also not allowed.
- Only men who have mild to moderate androgenetic alopecia (AGA) in the temple and vertex regions (ranging from III to V on the Norwood-Hamilton Scale) are eligible. They must also have ongoing hair loss.
- You have to revisit the center several times over 6 months, and apply the topical clascoterone solution on a daily basis.
- You need to have not taken finasteride or dutasteride within 6 months of visit 2. And not taken topical minoxidil within 12 weeks of visit 2. They need to be able to figure out only Breezula hair growth results via its androgen receptor (AR) antagonist mechanism of action. This requirement may dissuade a majority of hair loss sufferers from participating.
There are a total of 8 inclusion criteria and 14 exclusion criteria that you must read before deciding to volunteer.
Another major issue is that there are various Breezula clinical trial URLs floating around, and past company announcements were a bit confusing about locations. Moreover, even in the below two links, the location section is a scroll-through box where you can only see one location on the screen at a time.
So I am pasting all the 50 locations (across 4 countries) below in the hopes that I can help at least slightly speed up recruitement.
SCALP 1 Locations
United States
Arkansas Locations
North Little Rock, Arkansas, United States, 72116
The Petrus Center for Aesthetic Surgery and Hair Transplantation
Contact: Gary Petrus, MD
Contact: Terri Kim Rogers
+1 501-614-3052 Kim@DrPetrus.com
California Locations
San Diego, California, United States, 92123
Therapeutics Clinical Research
Contact: Neal Bhatia, MD
Contact: Hector A Bailon
+1 858-571-6800 hbailon@therapeuticsresearch.com
Florida Locations
Jacksonville, Florida, United States, 32224
Mayo Clinic, Department of Dermatology
Contact: Alison Bruce, MD
Contact: Matthew Dwarika
+1 904-953-3551 Dwarika.Matthew@mayo.edu
Miami, Florida, United States, 33125
University of Miami
Contact: Mariya Miteva, MD
Contact: Olumide Ojoola, MD
+1 305-243-8205 oxo160@med.miami.edu
Louisiana Locations
Baton Rouge, Louisiana, United States, 70809
DelRicht Research
Contact: Ira Thorla, MD
+1 225-412-0316 info@delricht.com
New Orleans, Louisiana, United States, 70115
DelRicht Research
Contact: Sarah Jackson, MD
+1 504-336-2667 info@delricht.com
Michigan Locations
Detroit, Michigan, United States, 48202
Henry Ford Health
Contact: Linda Stein Gold, MD
Contact: Fanar Razoky
+1 248-219-4304 frazoky1@hfhs.org
Minnesota Locations
Minneapolis, Minnesota, United States, 55455
University of Minnesota
Contact: Maria Hordinsky, MD
Contact: Jaime Nugent
+1 612-625-8625 speck007@umn.edu
Nevada Locations
Las Vegas, Nevada, United States, 89148
JDR Dermatology Research
Contact: James Del Rosso, MD
Contact: Melissa Jacobson
+1 702-964-2425 mj.drdr@gmail.com
New Jersey Locations
Berlin, New Jersey, United States, 08009
Hassman Research Institute
Contact: Michael Hassman, MD
Michael.Hassman@cenexel.com
Contact: Mark Capichana
+1 856-753-7335 m.capichana@cenexel.com
New York Locations
New York, New York, United States, 10022
Diane S. Berson MD, PLLC
Contact: Diane S Berson, MD
Contact: Kiera Murphy
+1 212-355-3511 staff@dianebersonmd.com
Stony Brook, New York, United States, 11790
Derm Research Center of New York, Inc.
Contact: Elyse Rafal, MD
Contact: Dawn D’Angelo
+1 631-880-9660 ddangelo@drcny.com
North Carolina Locations
Charlotte, North Carolina, United States, 28277
On Site Clinical Solutions
Contact: Catherine Pointon, MD
Contact: Alexandra Perez
+1 704-995-1198 aperez@onsiteclinical.com
Texas Locations
Austin, Texas, United States, 78759
DermResearch
Contact: Janet DuBois, MD
Contact: Hadie Mendoza
+1 512-349-9889 hadie@vialtrials.com
Frisco, Texas, United States, 75034
DelRicht Research
Contact: Brent Spencer, MD
+1 927-807-5787 info@delricht.com
San Antonio, Texas, United States, 78213
Progressive Clinical Research
Contact: Mark Lee, MD
Contact: Marta Reyna
+1 210-614-5557 mreyna@progclin.com
Georgia (the country)
Batumi, Georgia, 6000
LTD Health
Contact: Lela Beridze, MD
Contact:
Natia Bezhanidze
+995 597 51 47 34 natiabezhanidze3@gmail.com
Tbilisi, Georgia, 0114
JSC Curatio
Contact: Maia Datuashvili, MD
Contact: Neli Bakuradze
+995 599 90 23 54 Nelly_bakuradze@yahoo.com
Tbilisi, Georgia, 0159
Kanveni National Center of Dermatology and Venerology
Contact: George Galdava, MD
Contact: Victoria Sulava
+995 593 324 578 sulavav@yahoo.com
Tbilisi, Georgia, 0159
Tbilisi Cancer Center
Contact: Lally Mekokishvili, MD
Contact: Nino Kapanadze
+995 577 79 78 06 Ninucakap@yahoo.com
Tbilisi, Georgia, 0160
Aversi Clinic
Contact: Sergo Dadashyan, MD
Contact: Maka Katsia
+995 593 34 32 66 mkatsia@yahoo.com
Tbilisi, Georgia, 0160
Medi Club
Contact: Nino Lortkipanidze, MD
Contact: Mariam Kutidze
+995 595 80 19 89 m.kutidze@mcg.ge
Tbilisi, Georgia, 0179
David Abuladze Georgian-Italian Clinic
Contact: Josephe Kobakhidze, MD
Contact: Tinatin Shaishmelashvili
+995 558 50 75 55 tinatin.shaishmelashvili@gmail.com
Tbilisi, Georgia, 0186
LTD “Medinvestment”
Contact: Irakli Dartsmelia, MD
Contact: Tamar Loseliani
+995 577 73 63 33 tata.ioseliani@gmail.com
SCALP 2 Locations
United States
Arizona Locations
Scottsdale, Arizona, United States, 85255
Investigate MD
Contact: Brenda LaTowsky, MD
+1 480-440-5985 research@investigatemd.com
Florida Locations
Hollywood, Florida, United States, 33021
Physician’s Institute of Cosmetic and Reconstructive Surgery
Contact: Jonathan Weiser, MD
Contact: Wanda Bargot
+1 945-964-4113 wandabargot@yahoo.com
Georgia Locations
Atlanta, Georgia, United States, 30329
DelRicht Research
Contact: Robert Springer, MD
+1 770-417-4454 info@delricht.com
Michigan Locations
Troy, Michigan, United States, 48084
Revival Research Institute
Contact: Ali Moiin, MD
Contact: Sikar Grewal
+1 248-564-1485 grewal@rev-research.com
Warren, Michigan, United States, 48088
Grekin Skin Institute
Contact: Steven Grekin, MD
Contact: Claudia Attala
+1 586-759-5525 claudia.attala@adcsclinics.com
Missouri Locations
Wildwood, Missouri, United States, 63040
DelRicht Research
Contact: Christopher Kling, MD
+1 504-336-2667 info@delricht.com
North Carolina Locations
Huntersville, North Carolina, United States, 28078
Piedmont Plastic Surgery and Dermatology
Contact: Nicole Seminara, MD
Contact: Jeffrey Decker
+1 980-900-9229 Jdecker@onsiteclinical.com
South Carolina Locations
Spartanburg, South Carolina, United States, 29307
Advanced Dermatology & Cosmetic Surgery
Contact: Amylynne Frankel, MD
Contact: Ashley Davis
+1 864-574-0017 ashley.davis@adcsclinics.com
Tennessee Locations
Thompson’s Station, Tennessee, United States, 37179
DelRicht Research
Contact: Pezhman Shoureshi, MD
+1 629-895-1775 info@delricht.com
Texas Locations
Pflugerville, Texas, United States, 78660
Austin Institute for Clinical Research
Contact: Edward Lain, MD
Contact: Xiomara Duarte
+1 512-279-2545 XDuarte@atxresearch.com
Sugar Land, Texas, United States, 77479
Acclaim Dermatology LLC
Contact: Syed Ali, MD
Contact: Carina Espinoza
+1 832-770-6388 carinae@acclaimderm.com
Washington Locations
Spokane, Washington, United States, 99202
Premier Clinical Research
Contact: William P Werschler, MD
Contact: Celeste Gray
+1 509-505-1815 CGray@PremierClinicalResearch.com
Germany
Berlin, Germany, 10117
Clinical Research Center for Hair and Skin Science, Dept. of Dermatology, Venereology and Allergology, Charité – Universitätsmedizin Berlin
Contact: Ulrike Blume-Peytavi, MD
Contact: Annette Andruck
+49 30 450 518 458 annette.andruck@charite.de
Berlin, Germany, 10629
Emovis GmbH
Contact: Guido Burbach, MD
Contact: Julia Welle
+49 (0)30 439741220 julia.welle@emovis.de
Düsseldorf, Germany, 40212
Privatpraxis Dr. Hilton & Partner
Contact: Rodrigo da Mota
Contact: Melanie Momm
+49 (0) 211 86 29 28 90 momm@dr-hilton.de
Freiburg, Germany, 79098
Dermaticum-Privatpraxis für Dermatologie
Contact: Rolf Hoffmann, MD
Contact: Danuta Sobczak
+49 (0) 1702838441 dsobczak@t-online.de
Hamburg, Germany, 20095
Eurofins | bioskin
Contact: Walter Wigger-Alberti, MD
Contact: Sabine Schendel
+49 40 606 89 743 Sabine.Schendel@cpt.eurofinseu.com
Mainz, Germany, 55131
Department of Dermatology and Allergy, Johannes Gutenberg-Universität KöR
Contact: Petra Staubach, MD
Contact: Evelyn Mueller
+49 (0) 6131-17-2944 evelyn.mueller@unimedizin-mainz.de
Poland
Katowice, Poland, 40-081
Centrum Medyczne Pratia
Contact: Kamila Płaczek, MD
Contact: Paulina Majka
+48 721 201 421 paulina.majka@pratia.com
Katowice, Poland, 40-611
Centrum Medyczne Angelius Provita
Contact: Magdalena Kolanko, MD
Contact: Agata Puchowicz
+48 721 210 110 a.puchowicz@angelius.org
Krakow, Poland, 30-033
Centrum Medyczne All-med Badania Kliniczne
Contact: Grażyna Pulka, MD
Contact: Alicja Wolska-Król
+48 12 422 34 271 allmedpl@gmail.com
Kraków, Poland, 31-011
Centrum Nowoczesnych Terapii Dobry Lekarz
Contact: Malgorzata Dyczek, MD
Contact: Katarzyna Gajda
+48 690 000 367 katarzyna.gajda@dobrylekarz.com.pl
Kraków, Poland, 31-559
Diamond Clinic Sp. z o.o.
Contact: Barbara Rewerska, MD
Contact: Aleksandra Kubajewska
+48 571 241 412 a.kubajewska@diamondclinic.eu
Lublin, Poland, 20-412
Pro Life Medica
Contact: Agnieszka Sajdak-Wojtaluk, MD
Contact: Agnieszka Saja-Górecka
+48 537 827 727 a.saja-gorecka@etg-network.com
Warszawa, Poland, 02-637
Narodowy Instytut Geriatrii, Reumatologii iRehabilitacji im. prof. dr hab. med. Eleonory Reicher
Contact: Mariusz Sikora, MD
Contact: Marta Kurek
+48 603 315 033 marta.kurek@spartanska.pl
Warszawa, Poland, 02-661
Carpe Diem Centrum Medycyny Estetycznej
Contact: Weronika Mołga, MD
Contact: Paulina Litkowska
+48 608 888 888 “paulina.litkowska@gmail.com
Łódź, Poland, 90-436
Dermoklinika Centrum Medyczne s.c. M. Kierstan, J.Narbutt, A. Lesiak
Contact: Aleksandra Lesiak, MD
Contact: Małgorzata Szulc
+48 690 056 595 badaniakliniczne@dermoklinika.pl
Some of the names of the contact persons in these trials are very well known hair loss researchers. I am very optimistic about Breezula getting released in 2026 as projected by Cosmo Pharma.
I have mentioned in the past that a couple of hair transplant surgeons told me that they saw some great before and after results from a Cosmo Pharma presentation a few years ago.
Of course no-one expects a miracle. Perhaps something as good as Finasteride, without the associated changes in DHT, testosterone and estrogen levels. And a totally different mechanism of action (androgen receptor antagonist).
There’s even Rolf Hoffmann from Freiburg, one of the original founders of Replicel. I am not 100 % sure, but I think Replicel was founded in 2003 (!!!). Well here we are.
You seem very optimistic about Breezula, I always found it underwhelming – is there actual pictures available from the trials before?
Optimistic insofar as maintenance plus modest regrowth.
There was a before and after image on Twitter a while back, but I can’t find it anymore. Perhaps in relation to the below conference presentation from Cassiopea (see my Breezula writeup mid-way in that post):
https://www.hairlosscure2020.com/2018-american-hair-research-summit/
Otherwise, we just have their published Phase 2 results and their decision to continue to Phase 3. And (over)confidence at 2026 approval.
Winlevi acne results already seem decent.
FYI — they have been developing and testing Clascoterone for well over a decade. Perhaps not as old as Replicel, but I have not checked the patent dates.
Admin, I never saw the pics but my doc was excited about the prospect of Breezula as well. The odd thing about the trial was the differential between the 6 month and 12 month results but he didn’t seem that concerned when I asked about it nor did he offer a possible reason. I’ll send you a chart (which I may have gotten from you) showing the changes from baseline at both 6 and 12 months. Note the difference in 7.5% once a day vs twice at 6 months…..but then little more than 1 hair at 12 months. Since one of the changes made in the phase 3 trial design was lowering the concentration to 5% in favor of increasing the amount of solution applied (from 1 ml to 1.5 ml) for easier application, maybe they are thinking that consistency of application tailed off in the last 6 months. This is just speculation of course but results were still above baseline at 12 months.
Thanks Pinoitq.
For reference:
Below are 12 month target area hair count (TAHC) and target area hair width (TAHW) increase charts:
https://www.hairlosscure2020.com/wp-content/uploads/2019/04/Breezula-Target-Area-Hair-Count-Increase.png
https://www.hairlosscure2020.com/wp-content/uploads/2019/04/Breezula-Target-Area-Hair-Width-Increase.png
it looses 50% of his power on the 6 month mark…..sadly…but still works (i use it since almost 3 years)
Thanks for the shout out and reminding me that I have one foot on a banana peel and another in the grave Admin! ;-) Even if I was half my age and not excluded for being over 55, you are correct, your fourth bullet on not taking fin, dut, minox would’ve ruled me out. I wouldn’t’ve quit for the trial, bird in hand…
It is strange that they do not specify the 55 cutoff in their lengthy list of exclusions Yoda.
I would not quit my oral Minox and Dut for the trial either. Still hope that this post can help them reach their 1500 recruitment goal a few weeks sooner.
The age range is listed on Therapeutics Clinical Research’s site, they are running the San Diego clinical trail.
Thanks very much for posting this. I had been eagerly waiting for news on CB.
I agree people should not expect too much. A side effect free finasteride would be nice which slows loss and regrows a little, to tide us over until better advancements are available. Although I am doubtful it will be quite as efficacious as finasteride. I hope I can be proven wrong.
I do wonder why there is a cut off at >55? Do they have less confidence it will work well in people who are older with more advanced hairloss?
Any idea what will happen if this clears phase 3 in countries which were not part of the trial? Specifically here in the UK? Will it likely not be available as we were not part of the trial?
With that logic every medicine ever has or had to be trialed in every single country if you wanted to sell it as a pharma company.
I think that’s never been the case.
John, I have noticed quite a few trials have an age limit. If you read the Pleage research paper, they specifically tested PP405 for age related hair loss, along with stress induced and I think chemo. It may be that other factors, in addition to DHT, turn off the regenerative capacity of the follicle.
Do you think they are going slow?
Super initiative Admin. If underwhelming, at least we will know faster.
Admin, The Chart I emailed you is from baseline and not from vehicle……..so that you know the results were still above baseline at 12 months. The difference tracks so I think the chart I have is legit. It says that 7.5% BID was +4.7 at 12 months and that vehicle was -9.3, for a total differential of 14. I like that chart because it compares to baseline.
Thanks Pinotq, saw the chart.
I hope they release 6-month Phase 3 results and make these tables and charts a bit simpler if at all possible.
I will have been on the bootleg version for 6 months next Tuesday. I don’t want to jinx anything but I am still on it and just bought a lot more. I’ll fill you in when I feel comfortable that I can give a solid assessment.
Hey Pinotq. Thanks very much for your reply. That’s helpful. Yes I’ve heard age related alopecia may be a form of aloepcia in its own right and less affected by DHT/androgens. So I guess this makes sense of the limit.
That’s exciting news that you’ve been using the bootleg version and you’ve felt compelled to order more. I saw a video of someone recently who claimed to have been using up to 300mg per day and eventually went back to RU as he felt it wasn’t doing too much, however one can only take so much stock from these anecdotes given the uncertainty surrounding such products and their purity and vehicles. But it’s very good to hear perhaps some are getting good results.
John, The phase 3 Breezula trial is at a dose of 75 mg twice a day for a total of 150 mg per day. That’s the dose I am on. I have trialed RU in the past as well. Personally, I feel much more positive at 6 months on CB . I was off RU by this time. Trying to assess “maintenance” is a tricky game, especially if your stack already includes Dut and OM……and you have tried just about everything to come down the pipeline.
$110 a month seems a little extreme for such low results and over 12mo. Let’s hope that if is a side effect free topical that it’s popular and drives the price down.
Not to be pessimistic, but nothing too excited. I’m hopeful for other treatments. We need something that brings hair back we already have drugs that slowly maintain but nothing that stops hair loss. I am on 2.5 mg of Dutasteride daily since 25 years old when i was slightly receding. I am now 35 years old and have lost ground… I continue to recede and thin and recently added 5mg oral minoxidil. Being on the strongest stack out there and reducing dht by over 90% it still isn’t enough to overpower genetics. In my opinion if you have the baldness gene your genetics will eventually win no matter what current hair loss drugs you take. Lots of companies that are trying to create maintenance products that will mirror finasteride and Dutasteride abilities which is average at best. Looking forward to regrowth drugs hopefully some day.
Well said Tyler! I don’t know if you saw the video interview that Admin posted about Dr. Rassman but 1 line that jumped out at me was “……………the disease is progressive”. So I agree that a treatment that you have to take daily or twice daily, just to keep your head above water, is eventually going to be overtaken by genetics….different rates for different people. After countless years of treatments targeting DHT, it is refreshing to now see other prospective treatments working completely different modalities.
This. I’m glad there are products like this coming out but they can’t best genetics long term (heck, even a surgery isn’t always a cure as you can still lose the transplanted hair down the road) they just buy us time. Which of course is a good thing. Not knocking it.
The real cure is cloning imo (then transplanting the cloned hairs – the surgeons will love that) but I won’t see it in my lifetime. I guess we can clone a whole cow but cloning tiny hairs is too tricky :/
Hmmmm, I may look into this but I have scarring alopecia. I doubt this would do much. It may help the hairs I currently have. I’ve been using the AI bosley product you posted for about two weeks now.
I’m not that all excited, but it’s better than nothing I guess?! I’m sticking with topical FIN and oral MIN for the time being. I think it’s slowly helping, but it’s still early days. It certainly isn’t working on the frontal hairline.
I’m excited for you guys but I’m out of luck with anti androgen as they really mess with my heart. I took .25 MG of fin 3 days ago and my resting hr is 15 beats more per minutes then usual for three days straight. My chest hurts and im fatigued and have trouble sleeping. Gonna take some ibuprofen to see if that helps. I suspect fin gives me heart inflammation but I’m no doctor. I’m so bummed. Why do I even try anymore.
My doc said I could take oral min even with my heart issues (he said most can’t) but I had to stop my chest was killing me. Made my heart feel terrible. I told him and he said to stop taking immediately. Makes me wonder how good it can really be for you (though I know other cultures/countries have taken it religiously and have had no issues).
The thing is, min is great but a HUGE percentage of men over 45-50 in the US (many under that too, like me) have heart issues. Which means they likely won’t be able to take min. So yeah take the min…while you can. I have a feeling quite a few will have to switch to something else if/when they develop a heart issue down the road.
Also, I’m fit. Mine was 100% genetics and no diet or exercise would have prevented my heart issue (not the same for some…so stay fit to the extent you can).
Hi James, can your heart tolerate finasteride? Mine can’t seem to tolerate either fin or minox. This really bothers me.
Looks like a promising addition. Especially if it doesn’t cause sides which I’m concerned about and why I have yet to use fin/dut. I also bought some stock in the company. They are aiming to take over 35% of the market.
https://www.cosmopharma.com/investors/share-information