I first wrote this post on Winlevi for acne in 2019 and often update it when updating my Breezula post. This is because both products are made by the same company (Cosmo Pharmaceuticals) and contain the same key key ingredient in clascoterone. Winlevi is much less potent than Breezula (1% versus 5%).
Winlevi clascoterone 1% cream is a first-in-class androgen receptor inhibitor that can be used by both males and females to treat acne. In was first approved by the US FDA in 2020. Thereafter, it was approved for sale in the EU and Canada in 2023.
Winlevi was originally manufactured by Cassiopea (Italy), prior to the latter’s 2021 takeover by Cosmo. More product details can be seen here. The Winlevi for acne reviews on WebMd and on drugs.com are not bad compared to other drugs.
Update: March 21, 2024
Winlevi is the #1 Most Prescribed Topical Acne Brand in the US
In Cosmo Pharmaceuticals’ latest news release, it makes the following statement:
“Winlevi continues to be the #1 prescribed branded topical acne product in the US.”
- More than 927,000 TRx (prescriptions) have been given out since launch.
- Winlevi has been prescribed by over 17,900 unique prescribers, representing over 90% of total healthcare practitioners in the US dermatology sector.
Also of interest, Comso has initiated new commercial partnerships that have made Winlevi available in the EU, UK, South Africa, Middle East, North Africa and South Korea. And most recently, in Australia via Sun Pharmaceutical Industries (India).
Update: November 1, 2021 — Sun Pharma announces that Winlevi Clascoterone 1% cream is now available for sale in the US. To be used for the treatment of acne vulgaris.
August 1, 2021
Winlevi, Cassiopea and Sun Pharma
Winlevi (clascoterone cream 1%) will now be released in the US market in the fourth quarter of 2021. Official website here.
On July 26, 2021, Cassiopea announced that it was partnering with Sun Pharma in order to commercialize and market Winlevi in the US and Canada. On July 29, 2021, the company also released its first half 2021 results.
The US FDA approved Winlevi® (clascoterone cream 1%) in August 2020 for the topical treatment of acne vulgaris. This prescription drug is a topical alternative to Accutane and Spironolactone. It is an anti-hormonal treatment for acne that targets the androgen receptor. It works by reducing sebum production and scalp inflammation.
Many people will inevitably try to use Winlevi on their scalps for hair growth (via off-label prescription). This is not recommended by the manufacturer.
August 27, 2020
Winlevi Approved by the FDA to Treat Acne

Cassiopea’s Winlevi topical androgen receptor inhibitor was just approved as an acne treatment by the US FDA. It is the first acne treatment with a new mechanism of action to be approved in 40 years.
The US FDA is also reviewing a new drug application (NDA) for clascoterone cream 1% to treat acne.
More information from Cosmo Pharma (Ireland — COPN:SWX), which owns 45 percent of Cassiopea (Italy — SKIN:SW).
CEO Diana Harbort quote:
“Dermatologists have said targeting androgen hormonal activity in the skin is ‘the holy grail’ of acne treatment for both males and females.”
August 23, 2019
I have covered Italian company Cassiopea numerous times on this blog. Primarily due to its Breezula topical product for male pattern hair loss. Earlier this year, Cassiopea announced very good 12-month Phase 2 clinical trial results for Breezula.
Clascoterone and Acne
Cassiopea is also working on an acne product called Winlevi. Both Breezula and Winlevi are based on the same key ingredient: Clascoterone. However, the dosage in the latter is significantly lower at 1%. This acne product is almost ready to come to market per Cassiopea’s product pipeline.
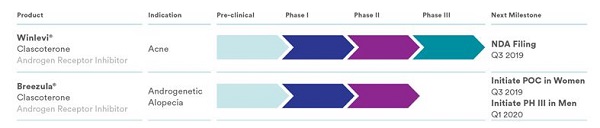
Earlier this week, Cassiopea made a major announcement. The company has submitted a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) seeking marketing approval for clascoterone cream 1% for the treatment of acne.
My Thoughts
Some interesting points in the press release and my thoughts:
- Clascoterone cream 1% targets androgen receptors at the site of application. This inhibits the local (skin) effects of dihydrotestosterone (DHT). Apparently, DHT is a key driver of acne lesion development. Most people wrongly assume it is solely testosterone.
- This acne treatment product will be safe to use in both males and females.
- If approved, clascoterone cream 1% will represent the first new mechanism of action in the treatment of acne in almost 40 years. Interestingly, if Breezula is approved for hair loss in 2021, it will be the first new topical or oral treatment for hair loss in almost 25 years.