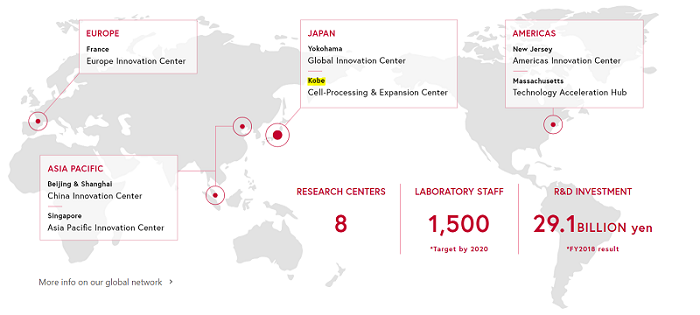
Update: Shiseido has updated its website. The Kobe cell processing and expansion center is now clearly visible on the map. Thanks for the link to commentator “Edgar”.
Shiseido’s Biggest Dot on the Map
Dr. Jiro Kishimoto’s Presentation
I last covered Japan’s cosmetics behemoth Shiseido in early March. This was in my post discussing Replicel’s two important recent announcements. For those who are new to this blog, Shiseido is working on a hair loss cure via its own research as well as via the use and rights to Replicel’s technology in Asia.
A few weeks later, we had the 11th World Congress for Hair Research in Spain. At this Congress, Shiseido (Japan)’s Dr. Jiro Kishimoto had a much anticipated presentation titled: “Autologous cell-based therapy for hair loss using dermal sheath cup cells.”
Shiseido 2019 Update
Several days ago, I got access to the summary of this presentation. Below is the most interesting and important section (with slight grammar changes from my part):
“After a Phase I clinical study with dermal sheath cup (DSC) cells in the EU showed no serious adverse events, we conducted clinical research studies in Japan on autologous cell based therapy for 66 male and female patients with androgenic alopecia using DSC cells. The study is currently ongoing at two medical institutions and no serious adverse effects have been reported thus far.
I would also like to discuss a unique registration law for cell therapy in Japan. This law enables hospitals and clinics to outsource cell and tissue processing to specialized cell processing facilities (CPC) located outside hospitals. We have introduced a cell manufacturing process for DSC cells in the CPC established in Kobe by ourselves, and I will discuss future prospects for cell-therapy in patients with AGA.”
At least we know this potential treatment hasn’t been throw into oblivion.
He talked about sides effects (which is good news), but what about effectiveness?
Thanks admin. I need a release date so I can book my ticket to tokyo and start making my wife fancy me again!
Does it say in the document when the trial ends?
Result for PHASE II in July, early July perhaps, according to Tsuboi.
So Shiseido is continuing forward and not stopping as we feared after the dispute with Replicel? And per the previous post on Shiseido, there is a quote that one well designed trial in Japan will likely lead to the treatment being approved.
But are these Phase 2 or Phase 3 trials? Didn’t Replicel finish Phase 2? I guess in Japan Phase 2 is same as Phase 3 now?
https://upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000026894
The results will be open in two months.
How can you tell?
I hope one of these Japanese companies comes through relatively quickly. I believe I’m one of many holding off on FUE until we hear something definitive.
These new procedures will take DHT out of the equation. From my research, it would not be wise to get a hair transplant now because these protocols are projecting a release date next year, and 2021 for Dr. Tsuji.
I’m only 18 and haven’t been using anything crazy like Fin., only minox and LLLT and a supplement – nutrafol. I’m not on Fin. because I have some faith in all this stuff, and I’m a pessemist by nature. My advice, should you choose to accept it – lol – don’t get an HT. The permanent solution (in Japan) is around the corner.
Hey, I did FUE and results have been great for past 10 years. It has given me enough time to not worry so much about losing my hair.
Having said that, I’m also really keen on seeing how this progresses. You can always do both…
In my opinion, if you are a good candidate for FUE you should go ahead and do it. Life is too short to depend on external actors to potentially solve your problems when you can do so yourself right now. Even if Shiseido comes out with a good treatment, it will be expensive and hard to obtain at first. You’re looking at at least half a decade before you can practically restore your hair via non-FUE methods. Literally the only downside to FUE, assuming you take finasteride too, is the cost, which can be mitigated by going to a reputable clinic in Turkey. Never do FUT though. Any doctor that says it’s better for you is full of it.
If Admin is optimistic about Shiseido’s optimism, then that’s good enough for me :-)
Glad to hear anyone talk positively about cell-based treatment. That will be the only true cure for 100% of bald heads.
Thanks:-) Though I have become lazy at looking through all my past posts to see what these companies were saying before.
For me, I found an un-bolded sentence the most interesting :
” I will discuss future prospects for cell-therapy in patients with AGA.” This strikes me as more to come not it’s a flop, but anything is speculation right now. And for those who are holding off on FUE, treatments, etc. thinking this will be readily available to the general public in the next year or two, with a one time trip to Japan then done, I don’t believe it will be that easy. Again, speculation on my part.
Very true. Based on past experiences, delays are the norm almost 100 percent of the time.
Right, but I theorize that this is not just a one time trip to Japan, at least two, maybe more. If you have the time and the $$$ to go multiple times in a few number or months/weeks then great. Also, I doubt that this will be a situation where it’s a approved and EVERYONE can start booking their appointment(s).
The idea is surely to centralise cell production and hair growth in Japan and let aestheticians everywhere implant the cultures on bald heads?
This sounds encouraging. There appear now to be reasons for optimism on several fronts. That said, I think my ultimate salvation probably lies with some sort of cloning or bio-engineering. On the latter, I wonder if we might have something on INVITROHAIR at some point? The web site looks interesting, and they had some sort of presentation at the recent Barcelona conference; but I can’t quite work out the difference between what they are working on and what Dr Tsuji hopes to launch in the next year or so in Japan. Thank you.
RCH-01 and Dr. Tsuji’s research ARE INVITROHAIR / cloning, as you know . DSC’s cluster to form new DHT proof hair follicles in higher injections dosages and Dr. Tsuji creates new DHT proof hair germs in a petri dish.
I agree with you. There is reason to be optimistic as these companies are approaching their deadlines. It might not be available to everyone at first – ’cause of $$$ and ✈️ to Japan but if you’ve got a plane ticket and some money saved up for the procedure, you’re going to be ahead of the game.
But there is a separate European company called INVITROHAIR which had some sort of presentation at a Barcelona Conference and has a patent for Haroid (I think). They seem to be working on the bioengineering of hair for transplant, which I think is different from Tsuji?? I would certainly consider a trip to Japan, but I’d assumed this would at first only be available for residents as they will need to monitor. But perhaps that is wrong. The sooner this is available in Europe the better. Am fast running out of road. Not sure being 18 means much as we all bald at different rates. I have lived with very slow balding for 34 years; but it is unrelenting! At least it didn’t all fall out when I was a teenager. I really feel for guys in that position. But as you say, lots to be optimistic about.
I see, INVITROHAIR is a different company but it seems like they’ll release later than Tsuji and RCH-01. Although, their work is interesting. Thanks for the understanding, truly. I can only imagine what it’s like to lose hair for more than three decades, but we live in an exciting, innovative time. Updates on these treatments are constantly coming out. Speaking of which, RepliCel say they’re on track here: https://mailchi.mp/replicel/may-2019-replicel-update
Also, I think that if Tsuji’s trials start next year and are successful, the procedure will be out in 2021 in Japan.
Lastly, have you considered exomes or umbilical cord stem cell injectoins? They’re supposed to be super effective. (But costly)
From a recent interview on Follicle Thought, and looking at their website, they claim to be quite advanced toward clinical trials and are applying for permits in the UK. As I say, they seem focused on bio-engineering of hair rather than cloning per se, but I don’t really understand all the biology. I believe there will be more on that site soon re the company. (Not heard of exomes before: will look into it, but am really saving my money for hair cloning as I suspect that it the only thing that will work for me. The next three years are going to be grim, as the front is going fast, but at least I now have some reason for hope: ….)
Admin, what do you think people’s reaction will be when RCH-01 and RIKEN’s protocol release?
How do you think it’ll affect the rest of the industry? Will people flock to Japan or stick to conventional methods?
This is the best time in history to go bald. Especially if you’re 18.
Hard to tell, but I am certain that the vast majority of people losing hair will not go all the way to Japan to try out a new treatment (I myself would wait a couple years to see 100s of results online and in person, and make sure there are no side effects with cellular approaches). Many won’t afford the initial price either I presume.
I think that for those who are NW3 or less, hair transplants will probably remain the most popular surgical option for many more years, even if they are told that hair multiplication is now a reality in Japan.
Adim, despite the costs and the travel, since these treatments are going to be one and done, do you foresee celebrities and public figures going to have this procedure done instead of HT? I’m not a celebrity but if those protocols give you a full head of hair period then I’ll fly to Japan and pay a lot of thousands for it.
“One and done”? Cell culturing and then implementation weeks later, at least two and done, maybe more. Who knows if one round gets us to where we want to be.
@Yoda Right, but after all the necessary weeks and months of culturing an injeciotns…it’ll be a permanent result then, correct?
Let’s hope so! I reserve judgement until we have the data.
Agreed. Results are key
It will be 3 travels for sure.
– First: for extraction and cloning.
– Second: for the first implant, with about 50-60 follicular units per cm².
– Third: around 6 months later, for the second implant to add another 50-60 follicular units per cm², to create the perfect density (100-120 units per cm²).
It’s not possible to create the perfect density in just one implant, that’s too much for the head.
Does anyone know why Breezula can’t be released in 2020? They should have finished their phase 3 trials by end of 1Q 2020. I would suspect this treatment will be much cheaper than cell therapy.
How will these treatments effect those with diffuse thinning all over? Even in donor area? My donor area isn’t the greatest :(
Like I said before, follow the money. If the other players had something better, I think Shiseido would have bought them instead of Replicel.
Sorry but off topic I bought a bottle of rogaine 3 weeks ago and the liquid started to crystallize. Liquid still comes out but I’m worried the bottle may be expired. Anyone have this happen and does it mean the rogaine is ineffective? I’m returning it to CVS but was curious if crystallization means expired rogaine ?
I’ve had this happen with gray market high-proof rogaine. It won’t work if crystallized. The minoxidil has to be in solution. Heating it up will usually help dissolve the crystals. Can’t imagine why a 5% solution should crystallize.
What about guys that have a good amount of hair that are on finasteride. Do they need to wait to lose all their hair before they could receive this?
No, you would keep your hair. They would just inject the DHT proof cloned dermal sheath cups into your existing hair and you can get off finasteride forever.
Thanks for the reply Greg. The words off finasteride forever is music to anyone’s ears. Obviously autologous therapy should be safe and so far so good.. this would have me crying tears of joy. Although im so scared safety wise I would let a few years go by just to make sure the people who are getting this done aren’t growing a tenth head lol.
Valid concerns Richieron but I’m pretty sure you’ve nothing to worry about with safety. If you can afford this treatment as well as the trip to wherever it’s being done you won’t need to do anything for your hair except cut it. Let’s look out for the trails though in July.
Hey Greg,
I read a study (I believe only conducted on mice) Finasteride changed Gliol cells. That certainly is not pleasant to hear have you heard of this?
Hey Richieron, I haven’t read the study but I’ll take a look. I know that finasteride can damage your endocrine system, make you infertile, make you lose your sex drive, and cause some brain problems. While none of these are very likely, they could happen. But one thing is for sure: it lowers your DHT dramatically.
From my research it wouldn’t be wise to go on fin. at this point. Updates about cell therapies, most talked about is Shiseido / Replicel are coming out very frequently. Check –> top of the page to view the latest. The real cures are coming. I wouldn’t take your chances with any serious drugs. I certainly am not and even if I did lose my hair completely, I never would. (But I won’t, and neither will you ;)
You’re the man Greg..
I have some legitimate things in my arsenal. I have mixed my own topical fin utilizing aloe vera film but place such a minute amount on my scalp im sure i have nothing to worry about. I was on Dut many years ago but got off, it worked very well. I just don’t think Fin or Dut are sustainable for anyone for too long for a plethora of reasons. I am with you I belive between follica, brezzula (off label winlevi 1st lol) and LLLT.. we should be fine until cell therapy.. thank for getting back to me my friend.
No problem, Richieron. We’re in this struggle together until it’s over. Topical fin won’t do anything to you, unless you’re 7 yrs old. I believe the reason all these creams and topicals – like follica and breezula – are coming out is because not everyone will jump to the “one-and-done” solution. For one, they’re going to be expensive! And second, if a product works to both keep and regrow hair, someone would be perfectly happy about using it twice a day. It’s a different market, yet it too has value.
Well said Greg.
Thanks, admin.
I would like to take the forum members’ attention to this active ingredient to treat hair loss (lactoferrin) as it has treated members of other forums.
Lactoferrin Study
https://link.springer.com/article/10.1007/s00403-019-01920-1
I note that Repunzel (Dr Christiano) now has a website: http://www.rapunzelbioscience.com/ Maybe still early days, but I assume this is aiming at regeneration rather than rejuvenation. Does anyone know? It looks as if they are going for gene modification as well. What with all the work in Japan, this, and the work in Europe (HairClone, and INVITROHAIR), things look as if they are really beginning to hot up.
Will it recover all follicles or just the ones that still have hair?
All follicles have hair. Follicles don’t die.
True. That’s why Shiseido’s method has the potential of being a 100% permanent solution with coverage of all your hairs.
Here’s something I found under a video regarding TISSUSE. I’m wondering if it has any merit. I also wonder if any of this, if true, is potentially applicable to Tsuji’s future treatment
“The science behind This new technology is shaky at best. Even if the new hair grows from the neopapillae, it would be under poles It would be uncontrolled growth. Meaning the hairs will come out from all different angles making your hairline look unnatural”
Sorry, scratch the “under poles” that was a typo.
Things seem much better than a long time ago but still its all just If’s and And’s. Time for the rubber to meet the road and get any of these products out that can actually be used This Year.
At least we have hope for this year with this news due out in a few months and Aclaris.
I agree. I the trial is successful they could release RCH-01 in Q3 or Q4 of 2019. The results of that trial is going to be pivotal, though.
As far as I can tell
Hair clone. *** 3 years
Shiseido. * 1 year
Tsuji * 1 year Dominant product
Follica. * 1 year
The too late to be in business list
Rapunzel. *****
In vitro hair. *****
Terskikh. *****
Tissuse. *****
POLARITYTE. *****
Aclaris. ****
By the way someone needs to mail POLARITYTE and let them know hairloss market is 1000 time bigger than the burn market and copy paste their stock price into ur email
The other thing to consider is the fact that Japan has laws which allow stem technology to be fast tracked. I think the UK is also quite flexible; but may not be so in the US where things could take longer(?) I hope we will see something in Europe (either UK or elsewhere) soon after Tsuji; as am not sure how practicable it will be to get this done in Japan. I suspect it will require careful monitoring over a period of many months. Let us all hope that it is successful and becomes the norm to supplement hair transplants. Many of us just aren’t suitable candidates as we don’t have sufficient donor hair. After that, I suspect there will be advances in use of donor hair follicles without rejection. That is the hope for diffuse thinners who may not have sufficient healthy follicles to clone.
@Alan J I know what you mean. Not only is my hair thinning diffusley (only on the top luckily) but my heathy hair is thinner than a spider’s web. Which of these treatments are you most looking looking forward to?
Mine is the same thin hair in the back my dad is the same way. Tsuji’s team clearly states they have control over the bulb size itself as well as unlimited cloning. For guys like us it’s a double down HI5
@Egghead That’s great news but I don’t want thick hair. I just want my crappy thin hair back. Personally, would you go for Tsuji’s method or Shiseido? (If Phase II trail is a success) Regardless of cost? And which do you think will co$t more?
Hi Greg, you may already have read this series of Q&As
https://www.hairlosstalk.com/news/new-research/hair-primordiums-tsuji-organ-interview-sept2016/ , but there is one question which covers people who have some thinning in the horse shoe region with AGA. The answer seems quite encouraging (in my case the hair in the horseshoe is thin, but seems to be holding on). If this works I am not expecting to have the hair of a teenager. But unless one loses all healthy follicles then the procedure should make a material difference, in terms of providing coverage. For those who have no healthy follicles (and tests are being developed to see if the follicles will provide on-going healthy hairs), then I think the next step will be human to human donation of cells. Recent breakthroughs in South Korea (to prevent rejection) point the way for that. That is obviously a bit further down the line. Good to see so much progress being made in different parts of the World: Asia, Europe and the US. A more globalized and interconnected World is clearly working in our favour. Let’s hope that recent trends don’t reverse the progress that has and is being made.
Let’s hope so. Sorry to hear about your horseshoe thinning. That’s a bigger problem but breakthroughs are being made with some frequency. Let’s wait for Shiseido’s trial results to release in less than two months and an update on human trials for Tsuji.
In the US ??!! Nothing happenned here since Fin my friend …except tweets …
hey , i just saw your comment. My question is: even the people with very strong hair loss, they still hav,a t least, 200-300 healthy follicles, so if they d take those, and can create unlimited hairs, wouldnt that be a permanent solution? In the worst scenario, maybe the person needs some sort of refreshing every4-5 years, but since hair cycle lasts 4-5 years, during that time, he could enjoy a full head of hair
@Egghead Thanks for the update! I’ve followed you for a while and trust your predictions. Which of these treatments do you think will be most popular? And do you think Tsuji will be out in 2020? Didn’t they postpone the trials? Anyway, we live in an exciting time and it’ll be cool to witness what other organs they can regenerate in the future.
I’m not a maverick guru my predictions with hair loss I failed real hard thinking Thorn Medical was legit lmao.
I think Tsuji will be on time, he is back by RIKEN, RIKEN is the japanese government. Admin knows the time line of delays better than me but I don’t recall Tsuji ever stalling his 2018 2019 on time and stating 2020 for the last 4 years?
That’s good to hear. lol thorn medical was a big whoop. Hopefully tsujis trials will start soon.
Egghead.. That summary os awesome. What is “dominant product”? How confident are you on the 1 year dates?
Thanks!
Confident? I wouldn’t be surprised if follica was out between 12-18 months.
Shiseido /replicel iwas very very skeptical of they failed 2018 past toxicology was safe but lousy results… but this private email from Ryoji announcing a release actually has me on the edge of my chair enough to be risking floating thousands on the stock.
Tsuji I’m just flat confident in. I think we absolutely will have 1 treatment in 2020 but whenever tsuji finally releases – its truly what I would define as. “The Cure” by all means what this blog is made of.
@Egghead I feel the same way. Both Tsuji, whose methods I am both fascinated by and confident in, and Shiseido have timelines and deadlines. The bigger industry / incentive here is organ regeneration – hearts, livers, eyeballs, legs – you name it. “Follicle Thought” believes RCH-01 will probably release later this year, said in their latest post.
PS, when do you think Tsuji will release? You said dominant product 2020 – what does that mean? When do you think it’ll be available in Japan to the public?
I will also consider risking buying replicel stock about 2 weeks leading up to the announcement to pay for the flight and treatment.
RCH1 dead they doing new research In Europe from scratch they only bloody Injector and try something new , It’s on foliclethougt
They only have bloody Injector
I agree with egghead predictions. I’m still banking on follica and SM being the new Propecia minox for the 2020s. Sisheido will be released but it will probably only be maintenance which is fine but this day and age we should have full regrowth treatments and baldness being an option.
AL Opecia- thanks for your reply. Half the bottle is crystal and the rest liquid. I also noticed my hair quality is gone down hill. Pissed. I returned the bottle and got a new one. Crazy how Rogaine crystalized.
Why do you believe Shiseido will only be maintenance? Those DSCs make your existing follicles express DHT-proof genes as well as potentially create new ones with the same characteristics. If that isn’t as effective, even in higher dosages, then Dr. Tsuji’s treatment will be the full cure. U think 2020 will be the big year? Have to wait for those trails but I’m putting my money on it.
Mjones was talking about a RCH-01 rumor that it will stop the miniturization but not give regrowth. It’s possible but the Shiseido scientist on youtube doesn’t express these views in the experiment and animation.
8:20 Part 1.
https://www.youtube.com/watch?v=oKRwlNuDMU0
All I’m saying is if you see Shiseido hit international news next 2 months Replicel’s stock WILL absolutely be deterministic arbitrage. They own that IP outside of Asia. No matter how diluted the stock is —marketcap is marketcap. 7m today — if it hits a paltry 200m (size of AgeX weeks ago) which is feasible in a 12B market (without a cure mind you) it could go 30+ times it’s price.
To all the “Dilution rebuttal bros” out there mktcap / circulation = price –period — the end. A 500m mktcap leveraged at 2:1 from 7 = ~140 times investment. Thats not a prediction it’s basic math in securities accounting. What is uncertain is the big million dollar question Does Shiseido have the cure? We’ll find out soon enough. If they have peach fuzz it’s off forget it.
If they have whats in this video….. if they have something THAT sensational AND it’s in American Nightly News. IF IT HITS 500M mktcap!!? with 2:1
5k VALUES TO 700k …………. RETIRED!!! PEACE OUT BABIES EGGHEAD IS OWWWT THATS A WRAP
I’m not saying its going to happen but if IF they have the buzz.. OH YEAH ONCE IN A LIFE TIME
@Egghead You mentioned “dominant product” for Tsuji? Does that mean you can get the treatment or does that just mean they have the product? They probably optimized the technology to form new hairs by now with the higher concentrations of the treatment.
Also, is there a way these treatments can change your hair color? Not as important but just curios.
I’m not an expert dude ..
But the fact Tsuji labs stated they have control of the “bulb size” means they can engineer an unlimited supply of modified follicles larger than the ones you had as a teenager.
To me that says follica follicum Sam shiseido basically everyone has to compete with that. they all might be great treatments but at the end of the day RIKEN has the nuclear check mate here.
I see. Their technology is superior to everyone else’s. Hopefully they’ll be on track for this year. If not, next heat then.
Hi egghead! Also consider buying replicel stock now somewhen. I am just wondering how long it would take to get their method approved outside of Asia, which determines basically when they would make cash out of their treatment. They successfully collected around 30 millions in debt, which can at some point just drive them into bankruptcy – so I guess they would depend on cash flows sooner than later. Also, if Tsuji would come to European and North American Market at more or less the same time, people would probably go for that rather than Replicels solution. Do you know whether the approving regulations for the two companies are the same? Would be interesting to know when we can expect Tsuji in Europe and North America, compared to Replicel in order to evaluate the future competition. I think its a great opportunity too right now, but a) depending on the results of shisheido and b) on the race into the European and North American market, where their IP applies.
I was on twitter when Replicel’s CEO (Lee Buckler) was asked if RCH-01 would be available by 2024. His response was that it seemed likely, but only a few minutes later the post was deleted and a new tweet said instead it was too early to tell and reminded the questioner they were only in the first phase.
If he backs off 2024, then you should not expect anything before that and only consider the possibility of perhaps 25-26-27-28-30 etc.
Yeah but Lee Buckler doesn’t own the “real” RCH-01. Shiseido owns and has perfected their technology. Buckler asked his followers to go to WCHR 2019 and ask Shiseido for an update – pathetic. Buckler and Replicel are out of the equation. Shiseido are the ones to keep an she on. And they’re almost here. Phase II trials release in a month – July.
Shiseido has licensed the technology from Replicel with the agreement stating ‘co-development’ but I noticed that within Rep’s ‘Upcoming 18 Months’ announcement they made no mention of beginning the next phase of their RCH-01 with the dermal injector so yeah, I think it’s fair to say Replicel isn’t going to be of much interest for us in the near-future which was my response to HänsenMcfly’s enquiry about the North American Market and *that* being 2024+ (3 Phases before Market Approval). Shiseido’s ability to fast track the process is why most of our hopes are in Tokyo and not say Berlin, Mexico, California, or Vancouver.
**They still can’t say whether or not their technology works on advanced hairloss which is shameful! The early promo video showed a solid 6 recover a full head. — Oh to be 2011 again!**
I know. It’s stressful not knowing if it truly works or not, but that’ll be answered by the Phase II trials which release in a month.
Replicel + Buckler don’t even know what’s going on in Shisedo so yeah, I don’t think they’ll be the ones distributing RCH-01 at first.
Hey admin, what’s your outlook on the timeline? Will anybody release next year? 2021? Or even pessimistically, this year?
Things are finally coming to a Head (Pun intended).
Aclaris will be reporting with photos (assuming it works) the results of their Phase II JAK trials anyday now. give it a couple of months. If it works then we are a breath away from a full set of hair IF NOT then I will do Shiseido as not anything will be on the horizon for years. Thus its either Aclaris or Shiseido in 2019? 2020? Tops.
Indeed. I have been on Fin for three years now, and Trinov since start of the year. Has not stopped my shedding (may work for others). Trinov is not cheap, but I will probably give it another two or three months. I assume you need at least six to assess properly. I will certainly try Shiseido as and when, though as mentioned above I increasingly think only regeneration will be effective given my state of loss. Tsuji still on track for 2020, so there is hope that this will be available in next few years. And they are not the only ones working on regeneration. Wish I understood the difference between cloning and bio-engineering, as the latter looks as if it might come on tap at the same time or even sooner. Regeneration will be quite pricey (at least at first), so start saving.
Looks like the reign of finasteride is coming to an end, if you’re willing to pay for all this. (I am personally) I think it’s the best time in history to go bald.
where did you read that results for aclaris are coming anyday now??
How can Aclaris release their product by 2020 though????
I still dont understand why Breezula cant release their product quicker? Phase 2 results shows it clearly works and they only think they need 6 month phase 3 trials
What I understand is that it rejuvenates miniaturized hair, if you have miniaturized hair terminal (hair with pigment) should improve it to its initial state. If you have hair (like the one you usually have in the rest of the face) I think it won’t do much. For those who are diffuse diluents I think it would be a fundamental therapy.
What do you mean? Miniaturized hair is miniaturized hair. You’re implying here that vellus hair is different from miniaturized hair and you’re also implying that intermediate or transitional hair will be the only type of miniaturized hair that will regenerate. If this is what you mean, can you send me your reference for this?
This news sounds good. Hopefully the results from Phase II will be successful since they have the technology, just upping the efficacy.
Also, does anyone recommend spending thousands of dollars on other current hair loss treatments in light of all these new permanent ones coming out? (Not transplants btw)
Aclaris is in beginning stages. Will be very long time before (IF) it gets released. NASA I love your optimism man but this is years down the road.
Lol we’ve been telling NASA that verbatim since 2015 that JAK lotion has been 6 months away from Walgreens in his mind for 4 years.
Just gotta step back and let him do his thing. Anytime we see the word JAK we all think about NASA
Hey admin, have you seen this? It popped up on my Facebook feed. This guy is a well-known hair transplant surgeon who is now offering this multi-step treatment plan for regrowth (not confident it’s legit though):
It’s a shampoo, man. Read it again: a shampoo.
Tsuji = 2024
Shiseido = 2021 and up to 10 trips necessary
The other potentially exiting breakthrough that could come any day is hair printing. There was a BBC article on this in October 2016, with much speculation then of at least three years away. But L’OREAL is already printing skin. I think we may possibly have this some time within the next three years: though it will no doubt be quite pricey, at least initially.
If Shiseido works it won’t be long before it spreads across the world. I bet after a year or two upon release it will spread like a wildfire. I can’t think of a more sought after cure, other than cancer and AIDS/HIV. The amount of money that would be made off a hair loss cure would be mind blowing. The North American market isn’t going to allow its customers to travel over seas for long. And anyways, you never wanna be the first person getting a procedure like this done, wait a year or 2 and see what happens.
@Wheeler I agree. They actually already cured HIV in one person 100%. It’s mostly younger people who care about their hair loss so this market only has room to grow. Let’s wait for those trials releasing in a little more than a month.
I read a study (I believe only conducted on mice) Finasteride changed Gliol cells. That certainly is not pleasant to hear has anyone hearf of this?
Hey Admin,
Do you know what ever happened to ASC-J9?
No :-( First time I even heard of that, although I could be forgetting.
It is being developed by Androscience it is supposed to degradate the androgen receptor topically and safely. However they have been in phase 3 forever. Havent heard much lately I will research more.
Seems like Androscience ASC-J9 is FDA approved to treat Prostate Cancer and in development to treat Acne. All this sounds very familar to other drugs researched to treat Androgenic Alopecia. They work on it since more then a decade. …
https://www.ncbi.nlm.nih.gov/pubmed/29203251
https://www.oep.com.tw/en-global/development_office/index
Good find John Doe!
Shiseido is soon going to blow the small players out of the water.
@Scott Big time. All this news about them signifies a launch. Phase II trials results will be international news. The real race is between Shiseido and Tsuji; all other players merely trail.
https://wakeup-world.com/2015/07/25/big-pharma-and-organized-crime-they-are-more-similar-than-you-may-think/
Pretty nice article if you have the time to read it. Sums up what we are dealing with and the factors in play with drugs.
Admin, if this works even slightly, for example this treatment give you back 10% of the hair that you need, but they are immune to DHT, do you agree that with some trips and money you can get back from nw 6 to nw 0?
I am nw 3 but I dont want maintenance.
Do you think that the treatment is working even slightly since they are not stopping the research?
Thank you
@Sandro Mastino We’ll have to wait and see about those trials. NW3 ain’t that bad. True, Tsuji’s technology will blow be a lot more effective but if Shiseido’s Phase II trials show a lot of regrowth in the higher Norwoods, it could give you far more with more time.
To answer your question (from my research and findings) The treatment won’t be maintenance, it’ll be permanent. They would’ve probably stopped research a while ago if they didn’t think DSC’s were worth investing in. They’ve almost begun marketing that new facility in Kobe – good sign, perhaps. (Check top of the page for info)
@Sandro absolutely! You would keep going back for them to plant another 10%.
Got this late.
What I’m suggesting is strictly money related ..their treatment absolutely would be stomped by riken yes. Replicel is a terrible stock and company on many reasonable levels. Buckles doesn’t even own any shares according to gurufocus. I’m not defending their short coming.
If their IP is successful its still going to take years for us to get treated in america period tsuji or rep. As far as money goes irrational exeburence will always outpace EPS fundamentals. Thats non debatable plenty of examples .. Last year One food stock appreciated 100%+ in one day just for renaming itself with the word blockchain in it.
If you have a legit cure with proof available in japan on world news under shared IP forget EPS fundamentals ..if there’s mania there’s mania and honestly I would say if it was a full on cure it would be reasonable logical mania.
I’m not long term investing this situation. I’ll simply float a position over July cheecking daily and pull out shortly after depending on how big the bang is… If shiseido flops its not like rep will go to zero in 15 minutes… they still have yofoto rcs wrinkles product coming to China etc so it’s not like a heart stopping rollercoaster trade for me personally. It’s an asymmetric binary float just in case its good news I profit.
@Egghead From your research, when do you think we’ll get conformation about Tsuji’s trials? From what I’ve found, they’re on track for this year or next year.
PS, nice description! You’re a good storyteller. Very visual.
Wrong. Shiseido wont bring a NW6 back to fullhead while Tsuji can. Also Follica and Ngf are also promising.
@NorwoodGuardian Who knows? Maybe it will. I wouldn’t bet on it but only the trials will tell. Either way, Tsuji’s protocol will demolish competition, if Shiseido doesn’t do so first. Look’s like Tsuji is on track for 2020/21. Shiseido will probably release late this year or sometime next year. The only reason they didn’t release last year was due to Replicel not holding up their end of the bargain.
Read the copy and pasted comment I posted here on a persons opinion on TISSUSE. After reading, I’d like to know what your thoughts on that Greg? Or anybody else. I’m interested and intrigued to know what you guys think. If what i sent has any merit, then you can probably kiss hair cloning goodbye. Just sayin’. Back to keeping my Galea loose any wearing a hair piece for 4 days out of the week. It’s a 100^ cure for me regardless that way. I don’t need to go out more than 4 days a week, I’m self employed.
@G Herbo What they’re saying about TISSUSE was resolved a few years ago when there was a breakthrough where scientists fixed those problems – some Italian group of scientists figured out how to grow hair that was aesthetically pleasing and looked completely natural. TISSUSE hasn’t partnered up with anyone big and still may be facing those challenges but as far as what we’ve seen with Tsuji and even Shiseido, these obstacles no longer apply. Their technologies have gone far beyond those fundamental challenges. I hope this clears up the picture for you.
Has somebody any info About this?
Almirall has finished Phase 3 and will launch soon.
The top-line Phase III results for ALM12845 (androgenic alopecia) in the EU show statistical significance
for the primary end-point (Change of Target Area Hair Count) at week 24. We expect the submission in
Europe of ALM12845 in Q4 2019.
https://www.almirall.com/documents/10876/3973413/20190513_PR_Almirall_Q1_2019_results_ENG.pdf/293613cf-18a0-47cb-961e-571763be699a
@John Doe : it’s polichem, the topical finasteride :) P-3074 (alternative name)
@ Left4bald Ah OK, so finally News for Polichem! :-)
… are there before / after pics?
Didnt these have all the same side effects as the oral version?
UCLA metabolite research moving forward:
http://newsroom.ucla.edu/releases/hair-loss-drug-formula-licensed
@PinotQ : really nice ! thanks !
Nice find PinotQ. Maybe our grandchildren will reap the benefits.
@Tomojones LOL! Come on, man. Have a little faith. Let’s focus on the bigger companies whose products are actually almost ready.
Will the use of any current treatment ( ie derma rolling, propecia, LLLT or minox) effect your ability to get any of these new treatments?
No. They’re probably designed specifically for people already using all that. Plus, the hair follicle is its own organ so its not really effected by anything you put or do to your body.
Even with the dermarolling? Or anything that punctures the skin?
I’m currently on nothing, early 30’s, just started to care. So I just need to last another couple of years right? I would say I’m a NW3.
@John Wicked Correct. Just another year or two until the actual cure. BUT, here’s a good regimine for now:
5% minoxidil 2x a day. (I recommend Lipogaine)
Tip: you could apply minoxidil everywhere where you once had hair. Who knows? You might regrow some there where there’s none or very little.
Capilus 272 laser cap (30 min every day)
Derma roller: 1.0 mm or 1.5 mm 1x a week and 0.25-0.3mm 2x or 3x a week. Make sure to wait a day or two in between.
Supplement: Nutrafol
All natural supplement that doesn’t significantly block DHT but does to some extent. Slightly improves thickness and texture and contains a bunch of vitamins: biotin, vitamin D, saw palmetto…
This is a costly and dedicated regimine but it’s what I use for myself.
When these procedures come out, you’ll probably have to stop all other treatments for a few weeks or a month (since you won’t need them) but dermarolling actually improves collagen production in the skin so don’t worry about damaging it too much. Just don’t go crazy.
When these procedures come out, you’ll probably have to stop all other treatments for a few weeks or a month (since you won’t need them) but dermarolling actually improves collagen production in the skin so don’t worry about damaging it too much. Just don’t go crazy.
Greg, thanks for the info. What about propeica? If no sides, should taking for 2-3 years really be that bad?
You think LLLT would work?
@John Wicked Propecia reduces DHT whether or not you have side effects. Your body adapts to lower levels of DHT. That’s why people crash when they get off propecia. If you’re not on it I wouldn’t suggest getting on it. I’m not, at least. I don’t think it’s worth it with these new procedures coming out.
As far as LLLT, you never know. It’ll probably help so down your hair loss – I think it slowed mine down. But you have to keep your hair short so the red light can get to your scalp better. It’s not going to give you fantastic results but it might help.
Hello there. Questions for everyone:
What do you think is the most exciting / promising treatment on the horizon?
Is there anything that is genuinely worth pinning our hopes on?
Is there a current Top 3 of Anticipated Future Treatments?
@Stephen C The top 2 are certainly Shiseido and Dr. Tsuji. Both are cloning procedure. Shiseido could release as early as the end of this year or next year and Tsuji could be out in 2020 but 2021 is looking more likely. You can certainly raise you hopes for Tsuji as it’s the full cure – it will give you an unlimited amount of hair at your desired thickness. RCH-01 phase II trials come out in a month from Shiseido. If the results are good then you can get your hopes up. If not, then you can still have a little hope in them because their technology currently lets you keep all the hair you have. But for those Norwood 5,6 and 7s out there Tsuji is the main one to look forward to IF Shiseido doesn’t prove to be successful in July.
The one downside of these treatments / cures for the next couple of years: $$$ and a trip to Japan. Hope this clears things up.
I reckon the third is Breezula (and cheaper option)
@D1 For sure. You’ll have to use it for as long as you want your hair but it will probably replace minoxidil and finasteride in the coming years.
Thanks, Greg.
I have read about both (on this blog), but I’m still not entirely sure if I understand.
My understanding is that someone would clone my hair (do they take a sample of hair? A blood sample?) and then clone enough of these for a transplant. If you need another transplant in the future, they do the same thing again, so you don’t run out of donor hair. Is that right?
What do you mean “Shiseido could release”? Release what? Results or the procedure?
Also, what do you mean by “at your desired thickness”? My understanding of transplants is that you can only achieve a certain level of density?
At present, I’m a diffuse thinner who relies on thickening products and sprays.
Sorry for the questions! I appreciate any answers or clarity that you can provide.
@Stephen No problem. Yes, they’ll take a few DHT resistant follicles from the back of your head and clone them over a period over a few months and then implant them back into you. I’m a diffuse thinner just like you (all over the scalp). But with Shiseido’s RCH-01 treatment that could release later this year or in 2020, with results for their phase II trial coming out in July, you won’t need more transplants. Their method makes all you existing hair resistant to genetic balding. So you won’t need to wait for your hair to fall out.
What I meant by “at your desired thickness” is that the second treatment,
Dr. Tsuji / Riken can control the bulb size of your hair and basically make it thicker while cloning it. With this technology, you could have hair as thick as a copper wire if you wanted to (not that thick lol) but certainly thicker than what you have. And an UNLIMITED amount of density since they’ll be able to clone as many hair’s as you need. With this treatment you’ll never run out of donor hair since thy can always create more. This treatment could release next year but 2021 is looking a little more likely – but only time will tell. The pivotal point will be when Tsuji starts his human trials. Then the treatment is one year out.
Even though these treatments will solve your balding problems for good when they release, they’ll probably be quite expensive and only available in Japan for the first few years, thanks to the freight train of the FDA.
If you like you can watch “Replicel RCH-01” on YouTube to look at Shiseido’s anticipated result. The results that release in July will confirm weather or not that animation is an accurate simulation of the actual procedure.
Hope this helps. The cure is almost here (kind of). You’ll have to make a trip to Japan and all that, but nevertheless the technology is almost ready and the accelerated Japanese regenerative medicine legislative pipeline will make it easy for these treatments to come to market rather sooner than later.
Agree,Breezula is a total game changer for any1 who is NW1 – 3 max, if it’s as good as they say – And it does seem legit
Especially ppl who are just starting to thin, bald etc, its a miracle to hold it off until a big win comes
I cannot understand why they cant release it in 2020 as well
@D1 Because of the stupid legislation.
Greg,
You seem optimistic about both options! Are other people as optimistic as you? Is your optimism based on facts and research?
Can you give me a basic, idiot’s guide to Sheisido’s treatment? Is it a topical lotion?
@Stephen These aren’t topicals. These are complex cell-based cloning procedures. Read Egghead’s comment on his predictions. I’m not the only one with optimism. Circa 2020 has been the “baldness cure” target for the past decade and there’s a lot of competition and incentive to meet that deadline. There’s a lot of research out there and I base a lot of my optimism in it. Here’s the video on Replicel’s treatment: https://m.youtube.com/watch?v=cCe5mg7X6zg&t=10s This is the desired effect. The phase II trial that releases in a month from them will be big news. There’s already proof that RCH-01 works to some degree; they just upped the dosage and concentration of the injections in this next trial.
Dr. Tsuji’s treatment is direct cloning of new hair germs using epithelial and mesynchamel stem cells. Basically, he and his team (backed by the Japanese government) replicate the process of hair formation in newborns. This technology is superior to the former but the former could also be very effective. Let’s war for an update from Shiseido in a month.
Lastly, updates are constantly coming out on these things. For me, it’s another reason to be optimistic. Check the top of the page to see Shiseido’s declaration of their newest faculty in Kobe Japan specifically dedicated to their hair loss cure.
Who else can make 2020 now?
Follica, Follicum, Samumed, and maybe one or two more are anticipated to release next year. Shiseido will probably be the most popular for those willing to put down the most money and have their hair back for good (IF the phase II results in July prove to be successful). Other products, including Breezula, will be for people who want to spend less and use an everyday or every week / month product to maintain their hair. The markets are different. It’s like HT vs minox and fin.
How can Follicum release in 2020? arent they still doing phase 2 trials?
Also Samumed dont finish their phase 3 trials til october 2020? what am i missing?
These are projections. 2-3 trials are needed to clear legislation, at least in US. Plus, looking at the efficacy of each product plays a big role as well. If a product is successful in only phase 2, it could launch.
Why doesnt breezula launch then?? It did 400+ patients in phase 2 and the results were stellar. Also the money to be made for first couple of years for first mover could be unbelievable
I guess we’ll have to wait for an announcement from them. They’re probably working on marketing prep as we speak. The legislation process is nasty. Not as nasty for drugs as for therapies and treatments, like Shiseido and tsuji but still – can take a while to move through all the paperwork.
You are too optimistic. Don’t make ppl disappointed your estimated timeline won’t happen.
I would like to change my hair colour. I have NW7 diffuse thinning with a little small horseshoe of resistant hair.
I hope so much that Tsuji could replace all my hairs fallen out with brown hair colour. The roblem is if I get Shiseido next year my blonde hair will be safe and if get Tsuji in 2021 it will look like s if I mix blonde and brown hair together. So I am pretty disappointed that Tsuji don’t give us an update about his timeline that we can plan. Human trial will Start in 2020 sadly so a 2020 Release is not that sure.
The big question to Tsuji is: will it be available for foreigners during the initial commercial launch?
I can predict: RCH-01 will be full commercialization also for foreigner :-)
Haha man you won’t have neither one of those luxury problems you’ve mentioned in that timeline! Though I wish it for you and the whole community;)
@Sileyman Both procedures will probably be available for foreigners. Shiseido and RIKEN – the Japanese government – are both smart enough to know that people from al over the world will have interest in their treatments.
Also, Tsuji clearly states that the hairs they culture return to the patient’s natural hair color since they start producing melatonin again.
——————
For instance, do you expect to be able to archive a higher density or the ability to control hair color?
Mr. Toyoshima: As you pointed out, our technologies enable us to control the density as well as hair color. Based on the results we’ve obtained, when cells used to regenerate hair follicle germ have no melanocyte stem cells, the regenerated hair will have no color. On the other hand, we can mix a cell population, which has been collected from follicles having melanocyte stem cells, to the cells used to regenerate follicular primordium.
————-
So my understanding is that they are able to control hair colour by mixing and adding pigment stem cell. The colour will result out of the mix of Eumelanin and Phäomelanin.
They have written in the Kyocera statements that the patient can choose the hair colour he wants so I don’t see your point that it only will be that natural hair colour.
Your thoughts of the full commercialization is very optimistic and sounds good BUT there are to different regulatory pathways SHISEIDO and Organ Technologies Are using.
SHISEIDO chooses the ASRM pathway which is full commercialization after Phase2
Organ Technologies(Tsuji) Are under The PMDA so they will go into conditional limited launch for 7 years and after that they go into full commercialization…
@Sileyman Agreed – I was saying that most people will want their original hair color. I know about the different commercialization pathways – since Tsuji is backed by the Japanese government themselves they probably have a firmer grip on him then Shiseido. Again, it will take longer for FULL commercialization for Tsuji but from the objectives he’s been hitting in the past years it’ll only be a couple more years before the procedure is available in Japan. My thoughts on Shiseido are optimistic but for a reason – they were supposed to launch last year, but they didn’t because Replicel didn’t hold up their end of the bargain with the trials in Europe. Plus, phase II results release in July. Whatever the outcome, the treatment could very will be out in the following months or 2020.
Okay, so if I’m understanding things correctly, the two most promising potential treatments / cures are:
-Shiseido / Replicel’s RCH-01
-Dr. Tsuji’s cloning procedure.
RCH-01 is supposed to stop any future loss, and potentially reactive hairs that are on their way out.
Tsuji’s treatment is a transplant with unlimited donor, and they can control the thickness of the hair they are transplanting.
What will we learn from the phase II trial update in a month? Is this a make-or-break update?
Does anyone know what stage is Tsuji at? What is the next trial or update or notable stage that will give us a better indication of where we are?
Also, which of these treatments would be better for diffuse thinners? If Tsuji can control bulb size and thickness, I would want thick hair implanted, but my concern is that my hair might look uneven, as the transplanted hair might be thicker than the native hair.
@Stephen C That’s correct.
Biggest players:
-Shiseido / Replicel’s RCH-01
-Dr. Tsuji’s cloning procedure.
“RCH-01 is supposed to stop any future loss, and potentially reactive hairs that are on their way out.”
“Tsuji’s treatment is a transplant with unlimited donor, and they can control the thickness of the hair they are transplanting.”
***
Phase II for Shiseido will be a major update, just as the phase I update was. It will reveal if their technology will really work.
Tsuji is at the preclinical stage. The announcement of his trial (which will be big news) will mean that his treatment is probably a year away. They’re planning to launch a trial in 2020, as they didn’t launch it in March, as they expected to. There’s a small chance they’ll start the trial this year but it’ll probably be 2020. Who knows, though? They might start this year. (but unlikely)
I’m not sure Tsuji’s treatment could do anything for your existing hair. It might have to go through the same shock phase as in hair transplants, but it is supreme to any other drug releasing (like the very talked about Breezula) or even Shiseido’s treatment. If you choose to get new hair implanted over your existing hair it’ll probably fall out and you’ll have a new head of hair with the characteristics you selected. But if you don’t care for hair color or a much greater thickness, plus if you’re NW3 or less you could go for Shiseido and have your exsisting hair back. Again, we’ll have to see about that trial in a month. But Shiseido would probably be favorable for diffuse thinners like us if their treatment is effective enough.
Cheers, Greg.
In some ways, diffuse thinners have the worst situation when it comes to hair loss.
Fingers crossed for RCH-01. I really hope it isn’t just a slightly improved PRP.
@Stephen C It’ll keep your existing hair (even the ones you can’t see) for sure; this they already proved in the previous trial. They really upped the dosage in this trial though.
Diffuse thinning really sucks. You have far less time on your head (lol). I think Shiseido would’ve dropped rch-01 a long time ago if the results weren’t there. In any case, Tsuji’s treatment will be the full cure and much more.
Stephen C, diffuse diluents have or retain most of their hair compared to localized alopecia (slippery baldness).
Treatments like dutasteride, they act better by the fact that they retain much miniaturized hair.
In case RCH-01 works as they say, many of these miniaturized hairs could return to normal, recovering their original thickness, that would be a great achievement for me.
I think RCH-01 will not do much for people with slippery bald areas. That’s why I’m hopeful in RCH-01. In case it does not work I would do another graft in the back of my crown leaving with a medium density, I know, that stinks but would not be bald if not a guy with more than 30 years with low hair density (something very normal in people of that age)
Dont believe in Greg’s timeline. It’s the best best case scenerio and the chance is extremely low. Not believe in me? See other treatment, if you can find one that is on time, i will say sorry to Greg.
I appreciate your concern for not getting people’s hopes up, but your negativity is equally destructive, if not more. There is nothing wrong for hope— Greg has his opinions and he backs them up with evidence. Nobody’s has the answers, he could be wrong, but you don’t know that.
Has it actually been verified that RCH 01 will make existing hair resistant to DHT?
From what I remember from the last trial results was that some of the gains made from the initial injections had been lost at the end of year 1.
Does anyone know whether RCH-01 works on a hairtransplant? I had a transplant in rest hair and it would be great if it could save those hairs, or even better, rejuvenate the hairfollicles under.
Okay, so if RCH-01 works, it will be a good thing for diffuse thinners?
Stephen C
If they work as they say yes! RCH-01 does not regenerate follicles, improves them.
@Soul Maybe it could. Let’s wait for the phase II trial to release to be sure.
I only say if it works as they assure in its theory….. It is still to be seen if this works as they say or as a somewhat more advanced PRP
@Soul There are stem cel therapy treatments that are already more advanced than PRP. Super pricy but the results are far better and last a lot longer. And those are jut stem cells. These are special types of stem cells. Even if the treatment may not be as effective in the first few months of application they technology is different and takes a longer time to build up density.
@Greg,
Do have a source/reference for what you say about the rejuvenated hairs becoming DHT proof from RCH 01?
Are in results supposed to come in today?
@Greg i read in an old article of 2016 that Tsuji aimed to start human clinical trial in 2020 and that he will be on the market in 2020.
Can you imagine that Riken will bring it instead after the human trial on the market without marketing? For me it is not sure to be so fast with human trial an commercialization within 1 year.
Do You think foreigners will have a Chance to get the treatment in the first year and why?
Thx
@Nafri If the trial starts in 2020, the treatment could very well be available the following year. As far as marketing goes, Shiseido has already started marketing their new facility in Japan and rch-01 isn’t even out yet. I’m not sure if they’ll bring it to market without marketing it. If the procedure releases it’ll probably be so popular that it might not even need marketing. We’ll have to see about marketing after / during the trial.
On that same note, a big chunk of the demand for the treatment is overseas so I think it most definitely will be available to foreigners. I personally texted Shiseido customer service about RCH-01 and they said it would be available to everyone who came to Japan. In fact most of the demand is probably outside of Japan.
The thing about these cell therapies is that they’re going to be expensive, comparable to hair transplants vs. minox and fin, except more costly. So even though Tsuji’s procedure will be popular it won’t be popular amongst all. Hope this clears things up.
This was the article:
https://asia.nikkei.com/Business/Kyocera-to-join-hair-regeneration-research-project
Anyone has opinions of Replicel issuing another round of public founding? Intersting enough the price started from 0,70 a share and ended at 0,40. Could be interpreted as a pretty bad sign, if not even shisheido considers getting those shares at 40 cents.
https://www.sedar.com/GetFile.do?lang=EN&docClass=8&issuerNo=00008892&issuerType=03&projectNo=02954269&docId=4577441