An interesting week in hair loss news warrants a wide ranging post.
Update: Make sure to read the comments from FAK inhibitor study co-author Dr. Kellen Chen in the comments section of this post.
FAK Inhibitor for Hair Loss
In recent days, a few people discussed this Stanford University research (first posted by “DrPhil”) on our hair loss chat.
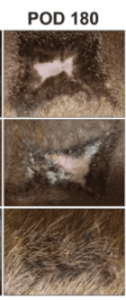
The researchers found that blocking mechanical signaling via FAK (focal adhesion kinase) inhibition promotes regenerative skin healing. Moreover, this restored skin includes hair follicle regrowth in addition to normal collagen fiber architecture. Most importantly, this hair regrowth was shows in both mice and pigs.
This is significant because porcine skin has striking similarities to human skin. The researchers used a pharmacologic inhibitor of FAK (FAKI) called VS-6062. This pharmacologic blockade of mechanical signaling resulted in skin with less scar formation and more hair.
The FAK was delivered to wounded skin using a biodegradable and biocompatible hydrogel scaffold.
Better than Verteporfin?
Per @DrPhil on our chat:
“This VS-6062 FAK inhibitor does the same thing as verteporfin, but it inhibits a target which is upstream of YAP. Verteporfin only inhibits YAP, while VS-6062 inhibits FAK (which controls YAP) and also other targets. FAK inhibitors can also be applied topically, with no injections required. Moreover, VS-6062 has already gone through Phase 1 and Phase 2 clinical trials to treat cancer.”
According the Stanford team, porcine serum FAKI concentrations following local treatment were almost undetectable. In fact they were less than 1% of the maximum tolerated human dose observed in a previous Phase 1 clinical trial. So safety issues are likely to be nonexistent in my opinion.
Interestingly, the earlier verteporfin research was also undertaken at Stanford, via the Longaker Lab.
Cassiopea Topline Results for Female Hair Loss
We are eagerly awaiting Cassiopea (Italy)’s Phase 3 Trials for Breezula for androgenetic alopecia (male pattern hair loss). However, several days ago, the company posted an encouraging update on its Phase 2 proof of concept trial for clascoterone use in female hair loss patients. Clascoterone is a topical androgen receptor inhibitor, and its hair growth benefits are almost certainly going to be greater in men.
The trials encompassed 293 women aged between 18-55. The were split into four groups: 5.0% and 7.5% twice daily application of clascoterone solution; versus twice daily 2% Minoxidil or vehicle. Only the subgroup with women less than 30 years of age receiving twice daily application of 5% clascoterone solution showed statistically significant differences from baseline in total hair count at month 6. No safety issues were detected.
Drew Brees Hair Transplant
Drew Brees is a superstar quarterback in American Football (NFL). The 42-year old retired in 2021 after a stellar career. He was also well known for his early hair loss and receding hairline…until this week. Looks like he got a hair transplant with excellent results.
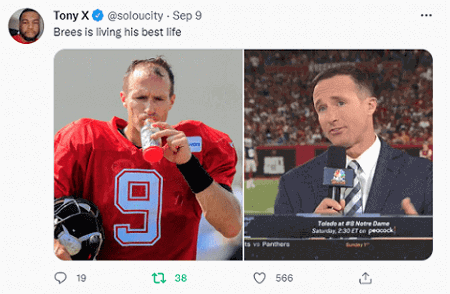
I have discussed numerous other celebrity hair transplant results over the years. Among the most famous athletes who got hair transplants include Wayne Rooney and Brian Urlacher. Possibly also Lebron James.