An interesting week in hair loss news warrants a wide ranging post.
Update: Make sure to read the comments from FAK inhibitor study co-author Dr. Kellen Chen in the comments section of this post.
FAK Inhibitor for Hair Loss
In recent days, a few people discussed this Stanford University research (first posted by “DrPhil”) on our hair loss chat.
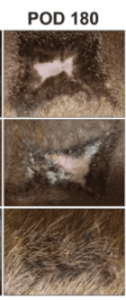
The researchers found that blocking mechanical signaling via FAK (focal adhesion kinase) inhibition promotes regenerative skin healing. Moreover, this restored skin includes hair follicle regrowth in addition to normal collagen fiber architecture. Most importantly, this hair regrowth was shows in both mice and pigs.
This is significant because porcine skin has striking similarities to human skin. The researchers used a pharmacologic inhibitor of FAK (FAKI) called VS-6062. This pharmacologic blockade of mechanical signaling resulted in skin with less scar formation and more hair.
The FAK was delivered to wounded skin using a biodegradable and biocompatible hydrogel scaffold.
Better than Verteporfin?
Per @DrPhil on our chat:
“This VS-6062 FAK inhibitor does the same thing as verteporfin, but it inhibits a target which is upstream of YAP. Verteporfin only inhibits YAP, while VS-6062 inhibits FAK (which controls YAP) and also other targets. FAK inhibitors can also be applied topically, with no injections required. Moreover, VS-6062 has already gone through Phase 1 and Phase 2 clinical trials to treat cancer.”
According the Stanford team, porcine serum FAKI concentrations following local treatment were almost undetectable. In fact they were less than 1% of the maximum tolerated human dose observed in a previous Phase 1 clinical trial. So safety issues are likely to be nonexistent in my opinion.
Interestingly, the earlier verteporfin research was also undertaken at Stanford, via the Longaker Lab.
Cassiopea Topline Results for Female Hair Loss
We are eagerly awaiting Cassiopea (Italy)’s Phase 3 Trials for Breezula for androgenetic alopecia (male pattern hair loss). However, several days ago, the company posted an encouraging update on its Phase 2 proof of concept trial for clascoterone use in female hair loss patients. Clascoterone is a topical androgen receptor inhibitor, and its hair growth benefits are almost certainly going to be greater in men.
The trials encompassed 293 women aged between 18-55. The were split into four groups: 5.0% and 7.5% twice daily application of clascoterone solution; versus twice daily 2% Minoxidil or vehicle. Only the subgroup with women less than 30 years of age receiving twice daily application of 5% clascoterone solution showed statistically significant differences from baseline in total hair count at month 6. No safety issues were detected.
Drew Brees Hair Transplant
Drew Brees is a superstar quarterback in American Football (NFL). The 42-year old retired in 2021 after a stellar career. He was also well known for his early hair loss and receding hairline…until this week. Looks like he got a hair transplant with excellent results.
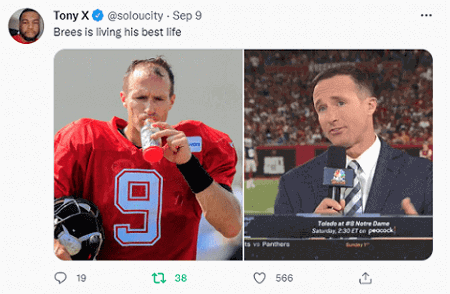
I have discussed numerous other celebrity hair transplant results over the years. Among the most famous athletes who got hair transplants include Wayne Rooney and Brian Urlacher. Possibly also Lebron James.
Nice finds admin!
The FAK inhibitors are intriguing. When you say less scar formation and more hair, does that mean more hair than than the surrounding areas? Because the picture looks like that will be the case.
I wonder when human trails will begin.
Hi, per our chat, someone said 50 percent hair regrowth. I did not see that number in the paper, but I read only 2/3 of it and just once :-(
Hello,
We observed about 50% hair and gland regrowth from our therapy compared to unwounded skin.
Thanks for your interest in our manuscript!
Since both the Veteporfin and FAK research came from Stanford, I sent some of the involved parties the below message:
https://twitter.com/HLcure2020/status/1437472366771073025
I am the lead author on the Gurtner FAKI study and also a co-author on the Longaker Verteporfen study.
Dr. Gurtner, Dr. Longaker, and I with all my co-authors are already collaborating heavily to potentially bring these pharmacological solutions to clinic!
Thank you Dr. Chen and great to hear! If readers have questions, maybe you can come back and respond here in next few days?
Hello Dr Chen.
Thanks for your research
How quickly do you think you would move on to studies with humans?
Also, many people seem to be speaking about donor hair regeneration, regarding inhibitors and a hair transplant. But is that possible? Because with an FUE/FUT you actually remove the graft, so in my opinion, it would seem that the hairs would not regenerate, because they have been already physically moved. I was wondering what your opinion would be on this?
Hello Patrick,
Short answer: we are trying our best to move on to humans as soon as possible!
Long answer: We have performed extensive studies using mouse models, but those are not good models for FDA. With our new publication on porcine regeneration, we now have strong preclinical data for FDA to move on to human trials. We are actually also in the middle of publishing on another large animal porcine study that will additionally strengthen our aspirations to get into human studies as soon as possible… stay tuned!
That’s a great question about the FUE/FUT. We unfortunately have not yet had the ability to test our therapy in combination with FUE/FUT just yet, but it’s definitely a great idea that we might try to pursue in the future!
Thanks for your interest in our manuscript!
I also want to clarify that we are first pursuing our therapy for wounds to promote healing, prevent fibrosis, and promote tissue regeneration. The ability to also use our therapy primarily to specifically target hair growth is an interesting future option for us.
Hello Dr. Chen
Is it long until pig studies with verteporfin will be published?
Also do you already know if they healed with normal skin?
– Magnus
That’s awesome, Dr. Chen. Thanks for sharing.
Do you have an idea how long it will take to potentially see these solutions in clinic?
Hi Dr. Bauder,
As you might imagine, these things can be quite variable. We are pushing for as soon as possible, but might take anywhere from 2-5 years from now.
Thanks for your interest!
I wonder if the FDA’s Accelerated Approval program would be a good fit for this drug. It might qualify, since scarring can be a serious and persistent medical condition that affects daily life.
The FDA says that they can provide informal guidance on whether a drug will likely qualify, so it could be worth discussing with them.
Dr. Chen,
I hope this email finds you well. I’m simply reaching out to see where things stand in relation to Verteporfin based solutions being available in clinic for scarring.
In addition, have you all seen improvement in scar healing when applying Verteporfin in conjunction with a FAK inhibitor.
Thanks in advance for your feedback regarding this matter!
Why is Cassiopeia’s update update encouraging if only a small subset of the women had significant results?
Per quote from Dr. Hordinsky in article and per their not ending the trials. It is unlikely that these treatments can ever benefit a majority of women as their hair loss is not as straightforward as male pattern hair loss.
It is also not necessarily a “small” subset. The max age was 55 and this group was below 30. Will recheck other sources later, but might be half the total or thereabouts benefitting.
In my experience, and after discussing online with other women with AGA, real AGA in women is pretty much the same as in men, only the pattern is different. Women with AGA who are successful getting regrowth get it from oral antiandrogens like spiro. I don’t get why a topical anti androgen would be more effective than a systemic one. Sadly enough I think it might even be less effective. Also, it makes sense that it works better on younger women since their AGA is not as ancient. Would be the same for men I presume.
When breezula phase 3 begin? It delayed over and over.
Impossible to understand why after the results of april 2019!! (if they are true) still waiting to phase III. I honestly think something is not true, they’re renouncing to all the money they could earn? We’ll hope it won’t be the same with Kintor.
So disappointed.
I have much more faith of eventually getting a legit lab/pharm compounding CB than Cassiopea phase iii ever going through.
HanBio again. The latest interview:
https://m.blog.naver.com/PostView.naver?blogId=hanbio_official&logNo=222505236540&navType=by
Nothing new at all. But at least their blog is active. Still everything looks cheap and fake. It’s very hard to believe. Would be great to conduct an interview via admin.
Thanks Ben. The new video interview of the CEO is available on YouTube too:
https://www.youtube.com/watch?v=qrhQi_hQvlc
I liked this comment underneath the video:
“Looking at the head of the chairman, I think it is not yet there. Please develop it quickly!!”
HanBio is a very small lab or company. They had only 7 employees with an annual sales of 1million in 2019. I think they keep issuing fake news or their hope in Korean media.
Is J. Hewitt‘s SHT project death?
Whilst the news about FAK seems interesting from my viewpoint, I do wonder whether it will be able to regrow long lost hair, or just be used to save existing hair from perishing.
Yet any form of potential cure is great news, as long as FAK won’t be a short-lived excitment like JAK inhibitors.
Hi Admin, I found two interesting studies, one is from this year April and affiliated with Pelage Pharma/Professor Lowry:
https://pubmed.ncbi.nlm.nih.gov/33739490/
“Hair cycle acceleration in these alopecia models led to the formation of histologically normal hair follicles within 30-40 days of treatment without any overt signs of toxicity or deleterious effects.“
I mean I am not 100 % sure, but if you can restore the hair cycle to normal, wouldn’t that be considered a complete cure? Sounds very alluring to me. Pelage the silent champion? Your opinion Admin?
Second study also very interesting with actual pictures (!) from a stem cell therapy. Coming from Italy, but already a little older.
https://pubmed.ncbi.nlm.nih.gov/28725654/
If you‘ve covered those already, apologies. Otherwise your expertise is highly appreciated.
Hi Ben,
I covered Pelage and Lowry here:
https://www.hairlosscure2020.com/lowry-and-lowe/
I also covered a few studies by the author (Dr. Gentile) of the second study that you posted in here:
https://www.hairlosscure2020.com/dr-pietro-gentile-hair-follicle-stem-cells-and-prp/
Hi admin, thanks for your response.
How can I dare to question your competence and thoroughness? ;-)
I didn’t connect your article with the Italian research.
But the Pelage paper seems to be new to me – is that probably the pre-clinical-study/proof-of-concept before a clinical trial which they did after the inception of the company and the funding of Allergan? Your opinion on that? Is my excitement too much?
I just saw on Reuter’s that scientists are going to clone a woolly mammoth by 2027 but we can’t have hair cloning?
There’s no way that’s happening lol
What ever happened to Follica? Aren’t they suppose to have the cure by now?!
FAK would be used with some kind of wounding I presume, like dermarolling? It always seemed the most plausible route, because growing from new is simpler than trying to undo existing damage, or work around it. And it worked on pigs which is a step up from mice!
It looks like Quercetin is a FAK inhibitor. https://pubmed.ncbi.nlm.nih.gov/29945484/
For a few weeks now I’ve been following microneedling (been doing that @2mo) with quercetin in emu oil. I’m doing half with quercetin and the other half plain emu oil. We’ll see…
Quercetin
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3273564/
Emu Oil
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4812284/
Thanks JFK for the comment!
Using FAKI to regrow from new definitely seems like the most promising option, and our findings in pigs definitely are giving us more confidence to move up to humans soon!
Alex, Follica pushed back another year. Trials resume in 2022.
Do you think future treatments can treat my diffuse thinning not just on top, but on sides too?
We don’t know if FAK needs surrounding hair to copy pattern and make new DHT resistant hair (likely does). The current best application I can think of for FAK is just significantly increasing donor hair. If all FUE or FUT sites could regrow 60+% of the hair follicles, then you can have a lot more DHT resistant donor hair with multiple treatments. I like this type of “cure” because the established hair loss industry will still be relevant. It will make them more money, so the HT doctors won’t secretly try to squelch it. If this works on humans, that’s a huge boost to viable hair transplant candidates. 60+% more donor hair is big news for many people. Hopefully the percentage might even be higher.
Makes sense. Harvesting the donor would create big wounds (compared to micro-needling) and make for a great cell signaling environment.
Hello Slick,
In our study, we were maximizing for tissue regeneration and preventing fibrosis, but it is possible that if we tried to use this therapy in combination with FUE/FUT, or if we changed the dosing/treatment regiment, we could optimize for maximizing hair growth to higher percentages.
Thanks for your interest in our manuscript and your ideas!
This year has been terrible. My hair is really going.
I like the idea of more donor hair. That’s my issue. I can’t get a HT – my sides are too thin and I can’t get enough from it to make a difference.
I hope 2022 brings something new. Anything that helps at least a little (though preferably a lot).
There is a brand new newspaper (September 2021!) with Henri Nico Doods (CEO of Hope Medicine) on the cover:
《进出口经理人》2021年第9期 卷首语 (tradetree.cn)
Trials For hairloss cure HMI-115(Bayer AG) Phase II planned For Q4 2021
Are you able to share the Informations?
Thanks Karl! I shared the news via updating the post, but have not tried to purchase the magazine and translate.
Where do you have the information from that the trials are planned for Q4?
Hi Admin,
Do you have an update on verteporfin?
Do we have an idea when clinical trials for scar healing with verteporfin might start?
No, I have not followed it much. I think the FAK inhibitors might end up being a better alternative.
Thanks admin, do we expect any clinical trials to be made for FAK inhibitors in the near future?
Except verterporfin could be used off-label today if the basic protocol was known.