Update: June 4, 2020
Follica Phase 3 Trials Scheduled for 2020
Follica (PureTech) just announced that it received positive feedback from a recent “end of phase two” meeting with the US FDA. h/t reader “Pier”. The company is now preparing to advance its lead FOL-004 androgenetic alopecia drug candidate into Phase 3 trials later in 2020. Official announcement by PureTech.
Approximately 280 patients with male pattern hair loss will be enrolled in the final Phase 3 clinical trials. Treatment efficacy will be assessed against two co-primary endpoints: visible (non-vellus) hair count; and patient self-reported outcomes on a pre-established scale. The randomized, controlled and double-blinded studies will be conducted across multiple centers in the US. A maximal use study to understand treatment pharmacokinetics will also be conducted in tandem.
Update: December 19, 2019 — Follica just announced positive topline data from its latest clinical study for androgenetic alopecia. There was a significant 44% improvement over baseline in visible (non-vellus) hair count totals after 3 months of treatment. The final Phase 3 study is expected to commence in the first half of 2020. Quotes from Jason Bhardwaj and Ken Washenik in there.
They did not mention which topical drug was used. However, I would not be surprised if it is just Minoxidil, based on the further below mentioned findings from last month. Hopefully, once the treatment is on the market, they can add more compounds into the mix without needing to go through clinical trials each time. A key quote mentions the use of an “on-market” drug, so no fancy newly developed products for the time being:
“Follica’s proprietary in-office treatment regimen combines targeted scalp disruption using the Follica Hair Follicle Neogenesis (HFN) device, with a topical on-market drug to create and grow new hair follicles.”
Hair Follicle Neogenesis (HFN) and Skin Disruption
One unusual section of the above press release mentions a comparison with other skin disruption devices on the market. The Follica HFN device “significantly outperforms other skin disruption devices” when it comes to hair follicle neogenesis.
However, this seems to imply that using any kind of skin disruption device can cause at least some brand new hair follicle growth. I should probably start at-home microneedling and dermarolling like tens of thousands of other people.
Follica’s approach entails generating an “embryonic window” in adult scalp cells via a series of short in-office treatments with its proprietary Hair Follicle Neogenesis (HFN) device. The scalp treatments last for just a few minutes, and they stimulate new hair follicle growth via stem cell stimulation. Following the wounding, a topical drug is applied to enhance efficacy by growing and thickening the new hair follicles.
Follica Might Start off with Minoxidil
Update: November 25, 2019
A few days ago, “Toccata” emailed me a pdf document in which one small part mentioned that Follica was going to use Minoxidil as the compound after the microneedling. Reference: How does Minoxidil work to grow hair?
Apparently, this document was also posted on several hair loss forums. However, no-one initially saw the page 46 small Minoxidil part except “Toccata” as far as I could tell. The new HLT thread on last week’s Follica news is already 10 pages deep. At this point, it seems like a few people have realized what was missed before and the pdf has been repasted several times.
I did not want to share this document right away. On Twitter, I first asked several members of the Follica team to contact me as I wanted permission to share the cartridge, dropper and new patient hair growth photos. In the past, several Follica staff members have responded to my requests and questions.
However, I got no response from anyone this time around despite two attempts on November 21 and 22. I did not mention anything about Minoxidil to them, so there must be another reason for the secrecy.
I have now found the same document available online here (edit: no longer working). On page 46, it states: “Proprietary Minoxidil cartridges for use with smart dropper”. Crazy to see this after years of speculation.
For many years, most of us have assumed that Follica would use a compound that would at the very least be Minoxidil PLUS something else. Many people assumed that the second compound would be based on valproic acid, since Follica even has a patent in that area. So this news is a disappointment.
However, two important points to note:
- The above linked conference call pdf was published around three years ago and is old news. Moreover, things may change further before the end of 2020.
- It will be much easier to get initial US FDA approval for a microneedling based treatment that only involves the addition of Minoxidil. After all, topical Minoxidil has been approved as a hair loss treatment for decades. Some people even take the more dangerous oral Minoxidil to treat their hair loss.
Correct microneedling and wounding in combination with Minoxidil seems to give excellent results (e.g., see Rachita Dhurat and her India microneedling work). Most people who are experimenting with at-home microneedling are not doing it correctly. Hopefully, Follica’s micro-needling device in the hands of a professional will be much superior.
Even more hopefully, Follica will add other compounds into the mix down the road. Perhaps even something like wounding plus mesotherapy with Dutasteride.
November 21, 2019
New Follica update via Puretech’s latest November 2019 presentation. (h/t “Toccata”).
Follica FDA Filing in 2020
They seem to be calling the product FOL-004. Previously they have used FOL-001, FOL-002 and FOL-003 in various clinical studies. The final name is supposed to be the acronym Follica “RAIN”. Note: The unrelated company Follicum labels its hair loss product as FOL-005!
Puretech has now raised its stake in Follica to 78 percent, indicating major optimism.
On page 32 of the presentation, it says that:
- “Interim clinical readout of optimization study in AGA” was completed in 2019. Checkmark next to it on the left stands for completion.
- In 2020, the company plans to get “Topline results from pivotal study in AGA” and also go through with FDA filing. Assuming data are supportive.
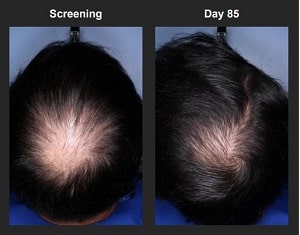
On page 40, they have a crown hair growth before and after photo. Pretty decent results in my opinion. It says the after photo is 85 days post the “screening” before photo. Not sure how many treatments were required over those 85 days.
The still use the phrase “newly created hair follicles”. Created via the wounding and microneedling procedure using Follica’s proprietary new device.
Apparently, the procedure will just take 5 minutes. Fingers crossed, maybe we will finally get this much touted product to market by the end of 2020.
The below post was published on May 12, 2019. See bottom of page for possible Follica Trial recruitment information.
Puretech Health Pipeline

A few weeks ago, PureTech released its 2018 annual report. On page 31, you can find the summary for Follica. PureTech owned 62.3 percent of Follica at the end of 2018. Follica also updated its site recently as noted by one of this blog’s astute readers last month.
In the above linked PureTech report, the Follica page mentions that the company will start its “pivotal study” in 2019. They have already conducted three clinical trials in the past.
Per one definition, Pivotal study can mean Phase 3 or Phase 2. Per other definitions that I have read, it means Phase 2b. Either way, we are making major progress. Considering that Follica’s methodology involves wounding and then use of existing drug compounds on the scalp, perhaps they can get away with a faster approval process.
I have covered Follica numerous times on this blog, only to be disappointed at its speed of progress each time. However, I have some interesting news to share about the company that I will split between two posts.
Recruiting Volunteers
While going through their new website in more detail, I noticed that one of their clinical advisers was a Dr. Jeffrey Dover. His was the only name that I did not recognize in the list. A little bit of research, and I found that Mr. Dover works at SkinCare Physicians.
I then went to SkinCare Physicians’ Twitter feed, and lo and behold, the below Tweet from February 14 says it all. I have no idea if this recruitment period is over, but please only call them if you are certain that you fulfill all the requirements, including travel to Chestnut Hill, MA). And do not call them just to ask general questions!
Update: Had to remove the embedded Tweet after a request from one of the company’s representatives. She emailed me and said that they were bombarded with a huge number of calls, with many volunteers not fully realizing the very specific travel requirements. A number of callers even pleaded to get in despite living in different countries! And of course many called just to ask general hair loss questions despite my explicit warning above :-(
excellent coverage Admin as always! Follica has us all excited obviously especially Mjones lol. Quick question does anyone know where to get legit Avodart by GSK without a prescription?
Does anyone know if using a wounding system could hinder the success of a ht down the track? I hope not cos up back and a ht up front could be the magic bullet.
Don’t ever ever ever do a HT. Not only do you get scarring in the back of your head, what they don’t tell you is you get scarring at the recipient site also, creating a cobblestone appearance. It’s awful and you’ll never be able to shave your head. HT’s ruined my life and my only hope is a real cure or stem cell unlimited donor hair. If you think bald is bad, there’s nothin worse than transplants.
Get lost. I had a hair transplant just last year. Best decision I ever made. I tell people all the time. DO IT. 90% success rate. You went to Turkey I bet, had a bad procedure and now your telling people not to do it.
People should really do it in America we’re the doctors in the greatest western nation are well trained. You leave little information. HT’S WORK. I am proof
Do you take propecia?
Turkey has some of the best HT surgeons worldwide, but if you chose to get a HT for several hundred bucks it’s your fault
No you get lost. They don’t look natural and the scarring I mentioned in the back AND the top is real. I’d take bald over what I have all day every day.
In the short term, HT is ok but you’ll end up needing more (donor) hair anyway, 2 or 3 years after the HT you will discover what I’m talking about. In my case (5 large HT with the best surgeons in Paris, NY etc…) I eventually lost my hair on top and no HT could help :-( I hope the problem will be solved for my kids in 20 years but for me it is a dead end. I guess the goal of 2020 is unfortunately a fantasy.
There have to be some HT successes on some very young early 20’s peeps that heal exceptionally, right?
Are you an HT doc?
Amen
David, what do you do now after the horrible surgery. Do you now just wear a hair system or have to deal with life with a hat on all the time which sucks during summer??
Great info admin.
Small gripe: The tweet is hilarious, it has a picture of a guy with a full head of hair frowning in the mirror lol.
Maybe you could argue his upper right hairline near the temple is diffusing a bit but this is a far cry from the state most of us are in lmfao.
I would say the picture is just a marketing technique to attract people who want to have a head full of hair like the model.
very fair point
Time to end the Griping and Misery as companies need to come out with something, anything, that works. My fingers are crossed for Aclaris and JAK and they will show photos assuming they are successful, or anything else that works.
Kobe Scientists Grow Precursors For Human Pigment Cells
https://scienmag.com/scientists-grow-precursors-for-human-pigment-cells/
Lol No no it’s fake news. dad said no good science can come out of Japan. Not until the Meiji restoration because their battery technology can’t save their debt to GDP. Trust me on this one.
Pivotal trial can be phase 3.. Why would they have an optimization study for a Phase 2b trial when they conducted 3 trials already? This is most likely their phase 3.
No, it is not.
The three trials so far have been phase I, IIa and IIb.
The pivotal trial is an optimization one and there should be a phase III, if they want to get an FDA approval and the green light to proceed with releasing their treatment.
Follica began in 2006 and it’s been 13 years already.
An effective treatment, that can be profitable and has virtually minimal side effects, is usually fast-tracked within less than 10 years.
I know it’s hard for most to come to terms with reality, but that’s the reality.
Follica’s RAIN treatment is BULLCRAP.
No. You’re wrong. If they conducted a phase llb already why would they have an optimization study for another phase llb? A pivotal trial is a trial that will have data to present to he FDA for market approval. Just look it up.
It’s an optimization trial for the phase III trial, pal.
Look at the logistics.
The pivotal trial is to last three months, do you really believe a phase III trial would last three months?
Bwahahahaha
Enough with this BS!
Wow you are dumb. There are exceptions to Phase lll just like any else. Everything they are using especially drug compounds are already on the market and are understood. You need to do more research buddy. Yes it’s an optimization study before the phase lll trial? When did I say it wasn’t? Get outta here with your bs already.
Chill guys , we all bros here
If you google minoxidil before/after results you find more impressive pictures than the one mentioned on p.40 of the slides. Sry for being the pessimist here but since this product contains market proven ingredients; it’s really unlikely that this comes even close to the bang we’re waiting for. Also wounding which is basically micro needling is nothing new. All in all it just seems like a more controlled version of these treatments which might be the only new thing here.
Excellent detective work admin! Thank you for keeping us in the know! Looking forward to your follow up post.
Bam Investigative journalism people
Hi5 admin
I just hope that after 25 years of waiting, the name of this blog corresponds to the truth … the end of baldness in 2020
“However, I have some interesting news to share about the company that I will split between two posts.”
There’s another post to come? You tease. Great find btw,
Haha maybe I should change that…second post is 50/50 at the moment depending on external circumstances. But it will likely happen sooner or later.
Hello Admin,
Any idea where I can purchase legit Avodart from GSK online?
As a blog owner, I cannot recommend unknown online sites (especially in other countries) for purchases of prescription drugs :-(
You can go through my main Avodart testimonials post and see what others have said about legit sites in the US…close to 400 comments in there.
Silly me of course.. sorry.. thanks Admin will do
Hi admin one last question do you know of anyone that has contacted Dr. Lupanzula in Belgium. I left him multiple emails no answer I wanted to inquire about his Topical liposomal Dutasteride. I’m wondering if he makes it in house or out sources. In that case I would just try and reach the pharmacy. Any input would be greatly appreciated.
No I never heard about anyone getting the product from him :-( I have never even emailed him as far as I can recall.
Ages 18-40?? Yoda calls ageism! :-)
Haha…maybe they won’t ask for your birthdate proof document!
Yoda looks 100 years old after 35 years of continuous, mostly high strength minoxidil use wreaking havoc on my skin…NOT! Just wanted to feed the trolls, I have awesome skin and told I look five to ten years younger than my actual “geriatric” age of soon to be 57. Don’t believe everything you read on the internet boys and girls!
Sorry but minoxidil does cause collagen breakdown that can manifest into aged skin appearance. Just because you’re lucky doesn’t mean it doesn’t happen. If you get off being a troll you’re a jackass.
The trolls are the ones who scare people off of trying things and exaggerate side effects Stevie. If minoxidil DOES cause aged skin appearance how could I be “lucky”? Can you please provide studies and photographic evidence that topical minoxidil causes “collagen breakdown that can manifest into aged skin appearance”? Or are just just talking out of your butt? Hence forward to be known as your “troll hole”! :-)
I love these guys that want to blame their aged skin, limp dicks and any other sign of aging on hairloss meds. Take care of yourself and you’ll forestall aging issues. Eat like crap, don’t exercise, drink like a fish, smoke, be unhappy and you won’t. Simple facts from Yoda, old man of hairlosscure2020 who still has hair after 35 years of the fight.
Haha! Btw there were a few people who commented in the Avodart testimonials page who were older than you for sure. Several over 60-years-olds have posted on this blog before, still hoping to get their hair back.
Trust me Admin, the hunger to have a full head of hair never stops no matter the age. Although it’s much more traumatic to be losing your hair in your late teens, 20’s even 30’s than in your “golden years”. This I know from experience, am grateful for the meds we have now that kept me from going full on bald and hopeful that the future will bring better treatments for all, young and old!
Two of my favorite examples of older successful guys who really wanted their hair back:
https://www.google.com/search?q=jim+traficant+wig
https://twitter.com/itvnews/status/982085466341208064
Trafficant…priceless, he was Trump before there was Trump, just a bit less of a douche bag. Although the Donald sets the bar pretty low! Here’s to new treatments and a new president in 2020! :-)
i am 55 years old, use 10 mg minoxidil oral for 20 years now…minox lotion doesn’t exists since 35 years, at least not in germany….i think i look younger than without the minox…..so same conclusion, better don’t belive.. even less/anything you read on internet…..
Follica is interesting. However why it sounds like every single company has to die for some years then come up with ” Uh-do-not-forget-us announcement”
Astute observation. Would be ironic if Histogen makes a big announcement next.
Replicel / Shiseido’s RCH-01 RESULTS RELEASE IN TWO MONTHS, according to email from their team’s doctor.
We live in an exciting time, everybody! Let’s not be so PESSIMISTIC, only observant. Past few years have been game changing, both in research and and steps toward commercialization. All this will be over before we know it. Either RCH-01, Tsuji, or somone out of the blue will grab all the cash in a couple years, if not sooner. Sure, all this stuff will be expensive but I’m willing to put down some serious cash to get my hair back.
Just think of all the other organs they’ll be able to regenerate in the future – hearts, lungs, livers, eyes… Hair is BS compared to the real industry in regenerative medicine. However, it is a gateway as it’s the easiest to replicate.
Sorry for such a long comment; I know this isn’t my blog but this $$$tuff is almost here.
Source?
I second this. Well said.
That is big news even if its a failure. If they are scheduling a news announcement that tells me they actually have something because why would you schedule out 2 months to announce a total failure? I doubt seriously it will rival tsuji complete regrowth and follicle size modification. But hey only time will tell good luck to us all.
Greg, Love the Replicel info.. Thanks for sharing! I hope that e-mail source is legit and you’re not just messing with us! I also hope they live up to their confident statements. It’d truly be amazing.
I know their was some people waiting for my reply in the tretinoin thread.. John yu, andy and mjones.. I have posted replies to your questions.
Thank you Quentin. I’m going to buy amino acid blend vitamin. I eat lots of healthy foods like avocado, dark leaf vegetables, legumes etc.
Nice find on follica admin. Let’s hope they pull through with nice density results so we can actually call this a real treatment and not just weak regrowth like 8%. If they can pull off 100cm2 terminal then we got good stuff coming our way. Time will tell….
https://www.hairlosstalk.com/interact/threads/follica-protocol-to-use-valproic-acid-patent-march-21st-2019.122520/
Follica new patent :
The main drug is Valproic Acid ! They talked about the drug applicator. They also talked about differents compounds as proteasome inhibitor such as lactacystin, a peptidyl aldehyde or pentoxyfilline.
Minoxidil is an optional and additional agent (or diazoxide or phenytoin).
” The drug applicator may be programmed remotely using an external or mobile device via Bluetooth or other wireless communications. In an exemple, the total dose is 1ml per treatment and is deliverediun five pulse of 0.2 ml each. The drug applicators maybe programmed to perform a Massage + Dispense cycle for 1 minute that is followed by a Massage only cycle for 5 minutes”
” Different drugs or compounds may be used including proteasome inhibitor such as lactacystin, a peptidyl aldehyde or pentoxyfilline (PTX) and the active ingredients descibed in section 6″
I know it says they are in Phase II or starting III but I have been all over the US Clinical Trials website (https://clinicaltrials.gov/) and can’t find anything about PureTech or Follica studies, has anyone found their phase trials posted there? If they are going for FDA approval doesn’t it have to be submitted on the clinical website?
Kinda off topic, but did anything of significance happen at the hair congress? I am guessing no?
JohnP I looked as well. Puzzling. Anyone know when they expect to actually begin the next trial?..my hope is follica will get me some regrowth then try and slow down the thinning with prp. Then annually go back and get the procedure done again etc..I dont think us non minox/fin users can do much else until samumed comes out with their product…hopefully.
What is the expected order and dates of these products coming out?
Sheisedo this year? Histogen this year? then Samumed and Breezula?
You guys need to flush histogram breezula and rivertown down the mental toilet with their flash on before flash off after I actually saw a legit Photoshop edit on (name removed) literally copy pasted false image for that brotzu crap.
For those of you new it’s basically Tsuji shiseido, follica ,Jak, in vitro hair, Tissuse ,Terskikh, repunzel and hairclone. And with great bitterness POLARITYTE since their technically already doing it but not making it available to us.
Some worthy pipeline side treatments might be follicum samumed.
Thats basically absolutely it.
Egghead your list is way too short! Yes there are a lot of disappointments and snake oils but there also are at least 30 more new drugs already in Human Trials. Hair Loss is just so complex to keep the overview.
Also dont Forget that beside Hair Loss drugs also Anti Aging, Stem Cells, Bio Printing and Regenerative Medicine is making fast Progress. These also are in Human Trials already.
What we all need is topical cream more than anything, which I believe should be possible
Breezula’s phase 2 were good. Why do you think samumed will be better?
Also JAK are taking forever
Histoscam I mean histogen lol gave up on that one.
I tried to sign up for the trial, but they are specifically looking for participants in the Massachusetts area.
The comment earlier citing the 13 year process for follica isn’t fair grounds to call it “bullcrap”. The average time span for a medication to go from concept to public availability is 12-17 years. I’d say they’re roughly on schedule.
LOL
It’s 15-20 years for compounds, which take time to be manufactured and be tested pre-clinically.
How on Earth would dermarolling be tested pre-clinically?
LOL
Enough with this BS!
Like anything else, with lab animals
ahahahahahahahaha
It was more of a rhetorical question.
There is actually no need to carry out pre-clinical tests.
We’re so f#$%ed…..jk I agree with egghead. Histogen, and ruvertown especially rivertown is scamville! Breezula is legit but 10 years too late in my opinion and it sounds like a pain in the ass to apply 2x a day. Diffuse thinners like me will look messy and sticky with it in my hair. We need next generation treatments like jak, sisheido, follica, riken etc. Like I said earlier I’ll still take anything just can stop loss and regrow.
Nice find Left4bald……….and the excerpt below may explain why the optimization study has taken so long………..more a matter of when to apply: [0009] In some embodiments, the valproic acid composition is administered once reepithelialization is completed, or 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, or 16 weeks after integumental perturbation. In some embodiments, the valproic acid composition is administered once reepithelialization is completed, or at least 2, 4, 8, 10, 12, 14, 16, 18, 20, 22, or 24 hours after integumental perturbation. In some embodiments, the valproic acid composition is administered once reepithelialization is completed, or at least 1, 2, 3, 4, 5, 6, 7 days after integumental perturbation. In some embodiments, the valproic acid composition is first administered 1 day after integumental perturbation. In some embodiments, the valproic acid composition is administered before and after integumental perturbation. In some embodiments, the integumental perturbation is performed after 24 or more hours of valproic acid composition administration
https://futurism.com/the-byte/grow-babies-artificial-wombs
Yet we still can’t grow a single follicle, but growing a baby in a lab is easier lol.
Lmao right?!
We can grow an entire new human from a Petri dish that lives forever but hairloss phew your talking a steep game buddy.
But jokes aside I must say in the last 6 years of haunting this blog I will say with all my cynicism the next 24 months are appear to be the strongest momentum in all the years I can remember.
Just noticed in your link to the study recruitment ad, at the bottom, it says that: “The study will last up to 12 weeks”. That’s seems like a short time period for a pivotal study (which I would think would require a little longer term safety data) making me wonder if this ad is for the optimization study or the pivotal study. Either way, the great news about that time period is that it sounds like they know very quickly how well the treatment works. Wondering if anyone who has called can shed any light on which study this is for.
I though of that, although since they started recruiting in February, I assumed that the study would not start for at least several months. In fact one person emailed me and said they are still recruiting, but only people residing in the same state. Making in sound like recruitment for a yet-to-start study, or one that started fairly recently. Could still be the optimization study if that one has not been completed already.
Thanks! It does say “new clinical trial”. Probably just speculative wishful thinking, but when they say the trial will last “up to 12 weeks”, it indicates the possibility to me that: 1) the treatment produces a noticeable result quickly; and 2) the treatment is in high gear and headed to market.
The king of hair loose research did it ! :)
I didn’t do this, but what’s the problem with asking general questions? I think it’s normal to want to know more about the drug in question that might be tested on you. I mean, they could just say they don’t/can’t answer certain questions. I don’t see how that’s rude or out of place but maybe I don’t totally understand how clinical trial phone lines work.
I strongly believe RAIN will hit the market like I have been saying. My only concern is will this work for people who have been using minox for over 5 years? Will this new treatment work long term for as long as you use it? Many people lose effectiveness of drugs like fin and min after few to several years of daily use. Finally, will it be 100 terminal full thick hairs or 25 per cm2. What is neogenisis hair? Newly created follicles that will go terminal after months of use?
Either way cots is my boy and I’m sure he will release something as he claims 4x better than what we have. I’ll take that for sure.
@MJones : According to their latest patent, Minox is an additional et optional agent.
The main drug will be Valproic Acid !
They talked about the drug applicator and about differents compounds as proteasome inhibitor such as lactacystin, a peptidyl aldehyde or pentoxyfilline.
Minoxidil is an optional and additional agent, if it’s not Minox they talked about diazoxide or phenytoin.
We don’t know for sure, for the results, but for now, it would be 25 hairs /cm2.
Neogenesis is for new follicle generated ! All your questions are in their patents.
I don’t understand how these newly created hairs don’t continue to fall out? Is there anything that says they won’t?
Will any of these be applicable for women?
Hi admin, You maybe interested in this study titled : 10-Year Finasteride Study: First to Investigate Long-Term Effects and Safety.
Here is an an overview of the study and a link to download the study as PDF :
https://www.bernsteinmedical.com/research/10-year-finasteride-study-first-to-investigate-long-term-effects-and-safety/
Interesting findings :
– Finasteride never loses effectiveness over time.
– The patient response at the first year can be used as an indicator of weather the patient is a good candidte to coninue with Finasteride.
– Patient over 30 tend to benefit more than others.
It is from 2012 FYI.
I was an excellent responder to finasteride. Stopped my hair loss at nw 1.5 for 13 years then one day my hair started miniaturizing again and scalp pains returned. So your study isnt accurate. …Propecia loses its power to fight of mpb over time.
There was mention of a split between two posts, what was the other post? This is the only near term interesting thing, than tsuji and tissuse.
Probably this from a month later, which turned out to be a bit disappointing:
https://www.hairlosscure2020.com/follicas-dr-dhaval-bhanusali-will-answer-your-questions/
Dr. Bhanusali was also going to give me some new updates, but never did.
Great news, finally something that works.
Wonder how it will work if used in conjunction with dht blockers such as finasteride and avodart.
I brought this up before…the company says NDA filing, FDA, pivotal study, etc. which makes you believe they are going through clinical trails however I have been all over the US Clinical Trials website (https://clinicaltrials.gov/) and can’t find anything about PureTech or Follica studies, has anyone found their phase trials posted there? If they are going for FDA approval doesn’t it have to be submitted on the clinical website? Unless they are doing their own private trials which I’m assuming means they could have released this earlier as a cosmetic treatment no? Unless unless they are doing trials in another country. Unless unless unless they only want FDA approval for the proprietary (hardware) device?
My guess is that because they are using existing compounds and a microneedling type device, perhaps they get away without having to do normal clinical trials. I am also confused about some of the terminology that Follica/Puretech uses.
It’s interesting, and it’s actually a matter of constitutional law versus regulatory power. The FDA has gone to court (and sometimes lost) over the matter. Obviously, valproic acid is prescription-only in the US. Topical use of valproic acid would be “off-label” use, which means it’s perfectly legal for a doctor to administer it this way, but illegal for a company to advertise or promote its use (to healthcare professionals and particularly to the public). However, pharmaceutical companies have successfully argued that it is an issue of freedom of speech, and that there is a difference between promotion and protected “communication” of facts.
Follica probably doesn’t need a new series of trials, provided it is armed with enough lawyers, and that its “communication” of the benefit of topical valproate (and its hazards) is “truthful and non-misleading.”
Moreover, it is apparently within the FDA’s power to simply add new labeled uses of a drug if it is satisfied with a company’s research without requiring full trials.
The result on page 40 is ok but you know that’s the best of the best .
Really nothing to get excited about
I’m just disappointed that we waited 15 years for that result. The fda needs to overhauled and more lax. Microneedling and adding valproic acid shouldn’t require 15 years of trialing lol. Anywho I’m not surprised that this is the result. Every new treatment is just going to be marginally better than the previous. Cb, sm, follica, all will have similar results. The real deal wI’ll be hair cloning but who knows if that will ever see the light of day. I also think follica will be good for bald spots. If you are diffuse it may rip or damage healthy hair. I don’t see the 30cm2 hairs they mentioned or the 100cm2 neogenic. Of course I’m just speculating here and they may release much better pics in 2020 and they are just low balling now for marketing purposes.
Mjones stop crying every time you come, you are the most tired that exists!! don’t you see the 30 hairs per cm2?? then come closer and count!! you really have the intelligence of a primate if you want to observe 30 hairs per photo!
Follica has announced many times that it will also improve the existing one! cielos sees a professional who treats your insane mind
Exosomes seem more promising
they need to show pics of NW5s…
Mjones good points. I think the next 2 treatments to come out will be very similiar to each other results wise. Keep in mind follica may be highly beneficial to the person that has light to medium hairloss. So let’s say you are the guy in the pic and your starting point is the “after” pic on page 40. Now that guy will go from “thinning” appearance, to descent head of hair. But anyone, let’s get this rock n rolling cause we need something now!
Admin,
Are you aware they are potential plans to run another pilot with an updated “device” ? I was told to monitor SkinCare Physicians’ Twitter feed for any potential updates , because if they follow through with the new device they will be looking for local volunteers located near Boston .
Thanks did not know that, even though I try to check their Twitter feed once in a while.
I’ve been following Gelesis’ (now called Plenity: https://www.myplenity.com) progress as it was Puretech’s first drug to be FDA approved (April 2019). I thought it was already out but turns out it will be 2Q 2020.
If we take *that* timeline into consideration, then an FDA approval of Follica in 2020 will have a market launch of about a year and a half afterwards. If the wounding device requires mass production and training, possibly more.
Meaning a more realistic release period is 2021-22, IMO.
And then there’s this bit: “Attractive physician practice economics consistent with in-office aesthetic procedures”
That has me worried, being that PRP needling is often in the $1000 range; even a shallow depth facial needling session is $300+. Follica most likely requires a lifetime dependence on in-office treatments (probably sold as bi-yearly packages, like 6 sessions 4-6 weeks apart, i.e. PRP), combined with a topical that will probably be $50-$100 a month, and we’re talking a yearly expense in the multiple thousands, and a whole lotta hassle, especially for maintenance-statistical improvements.
So I hope Puretech knows how to make this ‘attractive’ to us.
You are probably right :-(
I just sent you an email Admin. :- )
Thanks! Was a bit :-( news as you know.
If that’s the case, then lots of luck to them. In 2022, there could be two new topicals out, so why would anyone want to go through the added expense and hassle of a in-office treatment when there are other options out there (2022)?
That makes me wonder if PureTech even studied the competition well enough.
Rodri…..seriously? The way you respond back shows your intelligence level. I’m just stating the reality of it. If you find those results acceptable for 15 years of trials and delays then good for you. Enjoy. P.S. control your anger buddy. I have helped many newbie hair loss guys on here. Hooefully they took my advice the past few years and got on the big 3.
I don’t join to insult people or call them primates. You should see the mental therapist. I’m just fine.
I’m with Mjones. At the moment, this doesn’t seem that exciting.
For me, it looks microneedling and minoxidil. If it provides significant results and noticeable thickening, then fair enough. But if it offers minimal benefits, and needs to be done every three months or so, I’ll be disappointed.
Of course, results are key. They are the most important part. But practicality is important, too. Especially if results are minimal.
If a treatment can make significant, noticeable changes to my hair, I’ll put up with the hassle that comes with it. I’m sure most of us would. But if a treatment makes the kind of improvement that isn’t obvious, where you aren’t really sure, that’s not worth getting excited about.
Is anyone else excited about Samumed or RCH-01?
I believe RCH-01 is supposed to be a better version of PRP.
I don’t know much about Samumed. Can someone give me a basic explanation of how the treatment is supposed to work? I’ve read various articles, but a surprising amount don’t explain what actually happens!
I would be excited if Follica was a home treatment and affordable. No chance I’m going to an office for it for the rest of my life. What a hassle.
I predict Follica will have very disappointing sales results once the newest topicals come out in 2022.
If there 2020 top line results show that same guy’s bald spot filled in then I will be very happy. But if this is it then it won’t provide me much benefit. The little hairs that grew between the comb overs aren’t enough to make a difference. I go more regrowth using topical 82M that added density all over and grew hair along my hair line. It only lasted 4 months though. I read over at hlt that the follica results go back to baseline after 6 months. So this will be a continously needed procedure. Don’t mind that as long as it produces visible dense hair.
If it is a choice between Nothing and Follica I side with Follica. Life is getting short and the wait has been long I for anything that will grow hair that is out Now or at least in 2020. And, hopefully this will work all over the hairline.
I have said this before and it will make me the devil to most of you. Hair system, Norwood 7 to full coverage in 2 hours, guaranteed results visible to the naked eye, plus choice of hair colour.
The only drawback is it needs a re-glue at week 4. Perhaps a better adhesive that would make them last longer and more tolerable to heat would be the next hair breakthrough as my frequent trips to Africa and South East Asia have me avoiding them recently.
Do you still wear the rug Scott? Looked good on you when I saw it last?
Genuinely not sure what to make of this. And given Follica and puretech have been so secretive up until this point, could some form of subterfuge be entirely ruled out? I mean, 10 plus years and it’s rogaine plus wounding? Surely it wouldn’t have taken that long to put out, even at the FDA being their most obstinate?
Plus add to that that the first pics Follica have released, granted not widespread but put out there online for the lurkers to find, are not mindblowing paradigm shifting pics. The angle is different and it just seems that if Follica want to make a dent in the market, surely they know they have to have something better in their arsenal and I’m not convinced that they don’t either btw. And has anyone explained that new bit of info on the website:
CAUTION: Investigational device and new drug. Limited by United States law to investigational use.
Anyway, I get the feeling (or I hope anyway) they have more to reveal and are playing a bit of a long game here.
This is complete speculation but it may be that they have added the “CAUTION: Investigational device and new drug.” notification because they are planning to market the treatment, at least in the near term, without FDA approval………see this link stating how the term “Investigational” can mean off label use of an approved product for a different use or approved for use in a clinical trial.
https://www.fda.gov/regulatory-information/search-fda-guidance-documents/label-and-investigational-use-marketed-drugs-biologics-and-medical-devices. This could be either because the “device” does not yet have approval or because the compound requires a prescription…..i.e valproic acid.
I agree with mike. Mike im hoping this is a giant marketing gimmick to play with their competition. It just doesn’t make sense to have this little regrowth after all these trials and research….we shall see.
Mjones, why did you talk about Folica so much before? We all knew they have been delaying for 15 years or something right? it is just better absorbing Minoxidil using Dhurat findings I am guessing.
I’m most interested to see results in the frontal hairline region of the scalp. If they’re similar to the crown result pictured I’ll be very happy.
Mjones the guys on another site swear they are using minox. Please say it ain’t so??? I put a dab of minox on my scalp last night and my heart was racing the entire night.
Random question.
Recently, I’ve noticed that my facial stubble is not as dense as it once was. It isn’t patchy, but it was much more dense a few years ago. I’m 38 at the moment.
Same thing with my bodyhair. My legs and chest used to be very hairy. Not so much anymore.
So, I’m wondering if there is a connection with my diffuse thinning? Probably grasping at straws, but I thought it was worth asking.
Many years ago (ten years plus), I took Roaccutane. Could this have kick-started something?
Paul,
I have been suffering from diffused thinning as well for the last 5 years. It started right when I was taking Roaccutane.
At first, I suspected it. I encountered a couple of forumers whose hairloss seemed to start under isotretinoin as well. Eventually, I moved on and started worrying about how to prevent hair loss.
There could be something.
most probably follicas realized that something serious is about to come out … so they decided to take a step forward in an attempt to earn something … a bit like Brotzu and all the others …
https://www.dovepress.com/drug-delivery-system-based-on-minoxidil-nanoparticles-promotes-hair-gr-peer-reviewed-article-IJN#
Interesting potential boost to minox usage
I have to say I really dissapointed that after all these years that all follica has is a fancy micro needle device with minox. Its absurd. I read the literature and it’s TRUE, that is all it is. They should give half of all proceeds to Dhurat as she was first to discover this potential. So we have not made any real progress over at upenn after all these years and all these studies. The same people who cant tolerate minox get screwed again. It’s such horse sheet. I’m so ticked right now. Just really upsetting.
Has anyone experiment with using caffeine instead of minoxidil in concert with microneedling? Rachita also did a study claiming that it was just as effective on its own as minox. I’m thinking about that combination alongside some botox injections to the scalp because, apparently, scalp tension is a big contributor to hair loss.
Dogs are Good:
I’ve been thinking about caffeine + microneedling as well. I just started using topical zinc+B6+Epilobium Parviflorum+Oenothein B+Caffeine+Everclear+distilled water. This is a low-cost solution that should block DHT and provide growth stimulation with no side effects. I noticed the addition of Oenothein B and Caffeine has caused some scalp irritation, so I’m going to experiment with one-at-a-time to find which one causes this and reduce or remove it from the mix. Then I’ll start microneedling at 0.5mm and progress to 1.5mm as time goes on.
Current version of the solution (minus Oenothein B and Caffeine) takes away the scalp itch. I notice some very slight thickening of hairs at the hairline as well after one month of usage. Hopefully Caffeine is not the culprit in irritating my scalp, because I’m hoping to use it as a natural Minoxidil replacement.
Minox with microneedling?
Another one bites the dust.
These late developments show that aside from an accidental discovery the only hope is gene modification which is decades away.
Hair cloning is just hair transplant with unlimited donor hair and results will vary, not mentioning that most of us will not be able to afford it for a long time.
Follica, Aclaris, Brotzu. 2019 has been the year of eliminations, before a champion is declared next year.
@ Scott, Right! Also Taisho Pharma cancelled as well and Histogen back to Phase 1 … but on the other Hand there have been so many new stories as never before! I really want to hear more About Exosomes.
In my inbox today – perhaps nothing new for most of you:
“RCH-01 – RepliCel’s cell therapy for the treatment of Androgenic Alopecia has been the subject of a successful phase 1 trial in Europe and a clinical study now complete in Japan. Shiseido is expected to announce soon whether it will commercially launch the product in Japan or conduct further development and clinical testing. RepliCel will not plan for a phase 2 clinical trial of this product until it has the RCI-02 injector commercially available to use in such a trial and it has clarity from Shiseido regarding its plans for the product in Japan.”
I had an update about Replicel also:
“RepliCel is planning a Phase 2 trial to answer important questions like: what is the best dose (number of cells) to see intended results and how many rounds of treatments are required to achieve intended results. Trial success will be measured by assessing changes in terminal and vellus hair density as well as cumulative hair thickness **over a period at least one year.**”
So they won’t start a phase 2 until the Injector is released (2020-21), and when they do start the trial, it will last *at least* one year.
That’s gotta be around the five-years-away mark, not counting the phase 3 trials, ‘waiting for data’ period, registration and the rest of the inevitable delays. If Shiseido doesn’t come through in Japan, then we got Replicel 2030, if at all—they’ve been at this since 2003 and they still don’t know if it works—not good.
I agree with Admin that we shouldn’t jump too quickly to conclusions based on a conference call pdf over 3 years old. I’m not sure why minox plus micro-needling would even need FDA approval. There must be a reason why 3 years later, they are just about to begin their pivotal study. One possibility is that they are using a different compound (like valproic acid) at the time of disruption and then using microneedling/minox to sustain.
Microneedling coupled with minox. I dont know if I should laugh or cry. I’ve been waiting two decades and all cots is doing is trying to improve dhurats simple micro needle with minox regemin. So sad. He said almost a decade ago that another treatment is right around the corner. 10 or so years go by and this is what they have??? I just cant believe it.
Bill andrews with libella is offering trial of telomere age reversal treatment for 1mil
In Colombia cllaiming 20 years reversal
Tsuji. Tissuse. Rapunzel hairclone and maybe big maybe shiseido is all thats left.
Guys weve been here since 2014 … Topicals are NOT and will never be “cureworthy” its cloning or nothing dont get your hopes up for peach fuzz diffuse terminal treatments you have to squint to see or accidentally imagine
Agreed. I’ve always had that approach. Anxious to get an announcement from tsuji soon, looking Forward to TissUse trial in January.
Out in the real world: Guys getting incredible results with minox and needling
Here in the comments: Reeee you are doomed to baldness
Follica could pleasantly surprise yet, I remember back in 2017 follica tweeted:
Rox Anderson MD: “This isn’t simply a combination product, we’re growing a new set of follicles and talking to them”
How hilarious! As always the long drawn out trial to nothing more then minoxidil and microneedling! Hahaha! How hilarious! Ive been doing this treatment at home for 16 months now. Yes ive gotten decent results with definite regrowth but to pay all that money just to have follica do it for me is a joke. This is definitely not ground breaking. You want the same results? Go buy an at home microneedling device for a few hundred, and save yourself thousands. Another mediocere treatment.
Can you give me a link to buy? Too many type of microneedling device and i dunno which one is the best.
I like how the results state that the 44% improvement beats the historic 12% record for any other treatment.
So while this might not end up resulting in a YouTube video that “breaks the Internet” it has moved the needle in scientific progress.
It gives Shiseido something to compare against, with their undisclosed results. They must either at least match it or scrap their project.
Or maybe you can stack different treatments to further optimize.
What do they mean by 44% man. Is it the number of terminal hair increase. But they mentioned that they can generate new follicles. and percentage is a relative term… numerator/denominator.. if an area is slick bald and even 1 hair grows implies infinite percentage change. and what about the caliber of hair. did they take that into account as well. This is bs… How they have calculate 44%, they should be providing the calculation…. I can rest assure you follica is scam.. you cannot really measure the percentage change..
Better metric would be to measure how close the spot on scalp is compared to when it was not affected by MBP. Is that attainable, to what extent, how long.. etc..
and please provide the calculation…
_______________________________________________
And BTW
Rachita Dhurat is fake. Check her Google clinic page. she herself is rating and improving her rating. Here is the snapshot:
https://ibb.co/sbxKSRx
India is full of scammers. Even the big awards people get and journals that are published are based on high network, money and reach. Everything is bought. I have worked up the rung and seen enough boot-licking and mediocre doctors doing good just by the basis of marketing and networking.
Believe me, you can’t trust any things on the internet.
Even a small nick on one’s skin can result in scarring.
These temporary crappy cures are useless and such doctors are a curse. Ask them do such a cure on their family members and themselves. they will not. They know it’s all a marketing gimmick.
Getting patent and awards and all. It’s really easy.
Hell, even the most popular Indian film awards are bought.
The doctors at Follica – Dhurat, Bhanushali, Costarelis they are just milking the investors. It’s not their money that they have to put in. Just think about it, wth research are they doing… microwound and minox.. This kind of research will cost nothing in terms of time and money.
These guys are just usurping the money and will be awol very soon.
The problem is just because of such unethical people, there are other fake doctors coming up and promising such ridiculous cures and robbing people of their wallet, time, peace of mind and what not.
Man. Life is short. I know it hurts to see yourself turning less handsome. But that’s the game. Can you brave through this storm shit. If you can man, you can do anything.
Peace out.
In your snapshot, she rates herself once using her real name. And maybe it’s a clinic staff member who did that. Cheaters usually rate using fake names. Also, ibb DOT co is spammy per Google search so I will delete that link at some point.
Hi admin,
You are so active man.
If ibb is spammy, delete the link.
People can google and see if they wish to.
That rating was given more than a year ago…
Indian docs in general are technologically noobs.
so just that she rated once and forgot about the digital trace.
Anyway, I have peers who have written research papers: exploratory analysis, quantitative analysis, qualitative analysis and a lot of faff.
See, what you see in society in general is more prevalent at upper echelon in a more covert manner.
India is full of corrupt doctors, even milk supplied is adulterated. fake Godmen you would have heard about them…
Just employ Tesla’s concept of fundamentally breaking the things down and figuring out to how to do it better like in case of spaceX.
The study involves neogenesis of follicles. they can develop an AI model and simulate the work..
In practice, people will volunteer for the research…
you need to vary few parameters: depth, pressure, frequency and solution…
well whatever…my gut feeling…. follica…dhurat…bhanushali…costarelis are shady…
thanks for responding :)
peace out
Is this worth getting excited about?
Is the pic they posted the 44% increase or is that just a random pic? 44% sounds excellent on paper but if that pic is 44% then im not that impressed with my favorite follica:) Rogaine gives I think 16% and Propecia around the same regrowth. That pic looks like typical best case responders of big 3. I wish they would show a 2019 optimization study result pic not one from 2016. So much bs with these hair loss companies. My goodness.
Ken Washenik was on the press release…this means they’ll probably offer this treatment at Bosley facilities nationwide for quick expansion
. 44% ? ! I’ve seen 15% cover the scalp.
Pictures are louder than numbers
FYI From one of the news releases: https://www.businesswire.com/news/home/20191218005795/en/Follica-Announces-Positive-Topline-Data-Clinical-Study: “This process of hair follicle neogenesis involves minimal daily interruption”. It’s a guessing game as to how those at home might make micro-needling more effective but possibly once a week at 1.5 mm (per Dhurat) with daily disruption at no more than .5mm
Progress is progress. I’m glad to hear there are researchers pushing novel treatments down the pipeline after too many years, err decades, of no new options. I’m excited to see what 2020 brings!
Admin.. typo.. “They did not mentioned”
Thanks:-)
44% more hair in 3 months is FANTASTIC news. That’ll help a lot of people for sure. I didn’t get any real response from Minoxidil and am NW6-7, so likely won’t help me. Regardless, love the progress, and I’ll have a drink to this.
Maybe …. This guy in the picture day 365 might look better? But minox no thanks no way
Once more… 44% is a ginormous increase a 3 months! Now I’m getting greedy and want to know what it is at 6 months and a year. Also, what about slick bald areas that used to have hair? This progress is awesome! Great news. Thanks Admin!!!
I thought I would never ever say this but get me to Norwood 4 and literally all hats are off and into the garbage. No more wasting time on forums. No offence admin your doing us a great service.
No thanks on the minox…I still cant believe that after all these years all they have is refined microneedling and minox. No minox no way for me. The side effects suck for some. I’m really sad about this.
I want to know what 44% improvement in non vellous hair means and looks like. 44% total regrowth of scalp? Now that would be a game changer. Getting 44% coverage of a nw7. Or is it that you have 10 hairs then we gain extra 4.4 hairs? Any know what they mean?
Come on guys. Let’s be honest here: Follica is a joke. They’re simply trying to market current microwounding treatments by throwing big names behind a slick “proprietary” device and treatment regime. Assuming it gets FDA clearance, Follica will charge stupid money for it, and it will go nowhere. If you think this sounds overly pessimistic or negative, read their patent applications. There’s nothing special in them. No proprietary unique compounds. Plain old Minoxidil. Finasteride, Latanoprost, etc. all stuff you can use today with your needling device. It’s a shame how much attention & hype this company has received. I’m definitely onboard with the “more treatments, the better” mantra. But this ain’t new. This is just pure marketing 101.
it’s amazing how bitter and pessimistic balding can make men become. what does it matter if follica is using products already on the market, if their processes significantly improve their efficacy? show me anything else that can lead to a 44% increase in non-vellus hair count in three months. This will no doubt help a lot of men and tide people over as newer, better treatments become available.
@recedingdude – I hear what you’re saying. But it’s not bitterness or pessimistic to be informed and state facts. Here’s the patent number: WO2019232242A1
Point is there are real drugs & technologies being developed to hopefully effectively tackle baldness at one’s unique genetic level (Tsuji, RCH-01, etc.) Then there’s a lot of hyped up b.s. products that have come & gone. All the public evidence available suggest that Follica will be the latter. But believe whatever you want! Such is the nature of the internet…
No, all the public evidence suggests that have a treatment which can reliably grow a lot more via wounding than any other similar protocol that exists. Certainly more than most DYI ever see.
But believe whatever you want, such is the nature of the internet
“All the public evidence” being like two sources/examples, one of which is Follica themselves.
Or.. all of the public evidence like their latest patent application I’ve referenced (which you probably don’t wanna read) showing that they’re not really doing anything different from current ‘DYI’ rolling.
Or all of the public evidence of a company failing to put out a meaningful treatment for more than a decade…
The problem with your logic is, the current DIY rolling is not producing an average 44% improvement in existing terminal hairs.
If you’re already mostly bald, 44% of 0 = 0, so I understand your pessimism. But for those who still have some hair left, this is a promising treatment.
That’s not my logic, and I’m not being pessimistic either. I’m simply stating that behind all the hype and wishful thinking, Follica is just trying to patent a derma rolling program with minoxidil after a decade of talking a big game.
That 44% figure is not an average. And some people who ‘DIY’ derma roll experience a very significant improvement.
Follica trialed 3 treatments. The chosen treatment averaged 44% improvement.
“The selected treatment regimen demonstrated a statistically significant 44% improvement of visible (non-vellus) hair count after three months of treatment compared to baseline (p < 0.001, n = 19)."
Follica's studies were intended to optimize the intensity of wounding, and their chosen method is the optimum amount of wounding they found that results in hair regeneration. The wounding must occur in-office because it's far more aggressive of a procedure than at-home microneedling.
Yes, some people have gotten great results at home, but the average result is not nearly as good as the average result in Follica's optimized treatment.
Wounding without minoxidil results in new hair growth (and stimulation of existing follicles) but the new follicles quickly miniaturize over time. Adding minoxidil provides the follicle with a boost that allows it to go terminal and remain so. The best results will come from antiandrogen (Breezula) + a growth stimulator (Minoxidil). Those who are sensitive to minoxidil can use an alternative growth stimulator (numerous potential stimulators exist, and this will most likely require experimentation by the DIY community).
The treatment works best in areas that already have some hair (works similar to quorum sensing). Thus, the more your hair fills out, the more new hair you will be able to grow over time.
I realize many people are disappointed by the treatment, but it is a welcome addition to the battle against baldness. A treatment, which can improve existing density by an average of 44% is a really big deal to anyone who still has some of their hair left. That said, future enhancements will produce far better results and expand the range of good responders to treatment.
Exactèment!
Spot on!!!
I think the amazing point here is that, while minox alone created a small increase in visible hair by stimulating dormant hair follicles, this wounding technique is claiming to create whole NEW FOLLICLES. If you’re seeing 44% increases in hair density at 3 months by creating new follicles, then the potential here is enormous. The question remains on why new follicles would be DHT resistant. Then again, maybe all new follicles are DHT resistant for a set number of years (they were when we were young). Who knows?.. but new follicles are being created, and they’re growing hair at impressive rates. This is fantastic.
IMO, the new follicles are not DHT resistant. If Follica uses a DHT inhibitor in its topical it won’t matter so much. If they don’t, the treatment will be greatly improved by the addition of Breezula.
No matter how you slice it, an average of 44% increase of existing terminal hairs is an outstanding treatment. And for those claiming you used a dermaroller and Minoxidil to get the same result, you’re FOS unless you’ve gotten > 40% in the first three months. Some people have but the average among users is way less than 40%.
This treatment is an improvement to current dermarolling technology. If it were simply high priced dermarolling + Minoxidil, the company would fail. They’ve been experimenting for years to find the best protocol, and they’ve backed it up with scientific evidence. Even if it’s simply a marketing strategy, it’s a treatment you can’t purchase at home (prescription medicine required) backed up by the science of getting the absolute best result. Because we are talking neogenesis (new follicles instead of stimulating existing follicles) this is a game-changer for most people. Everyone will be doing it (in one form or the other) until a better advancement comes along.
This is a big deal. We will soon have a no-side-effect topical anti-DHT (Breezula), current stimulator (Minoxidil), and new follicle grower (Follica). The guys just now starting to lose their hair have it made. The guys already slick bald will need to wait longer for something better. The guys in-between will have major improvement.
Ladies gentlemen, this is a big deal to those that can tolerate minox side effects. For those that cannot, it is very disappointing. Follica will make a lot of money off of this, let’s be honest, as people are desperate to maintain and grow hair. It wont grow dht resistant hair but you will be able to maintain for the most part by using minox and their device. I really wish they developed an alternative to minox, even if it werent as effective, for all the people that have heart issues or get the nasty eye bags or bloating or low blood pressure. So disspointed for my self but happy for those that can tolerate minox.
So in other words in last 10 – 20 years we haven’t registered any progress yet. We’re still limited to our forever lasting Minoxidil plus needling. So it took several years for them to come with this? For how long Follica was developing this stuff? Why would it take lots of years for.. Minoxidil + microneedling? I thought they were suppose to work on sort of new “cure”. Ok it gives better results than other stuff on the market right now that s something better, but the idea of progress it s completely gone in this circumstance. To me it looks more like marketing stuff at its finest. Combine 2 things that already existed, find out the most optimal use, sell it under your license and call it a day.
I m thinking. What if they actually wanted to bring something completely new but in all these years they just failed so thinking that their boat was sinking they decided to combine these 2 things already on the market in order to save something from their failure and save their business at least?
No one knows Follica’s protocol. People are jumping to conclusions. I get sides with Minoxidil. I’ve seen plenty of people get good results with dermarolling and topical compounds other than Minoxidil. The reveal of Follica’s skin disruption protocol will help all.
There is absolutely nothing on the press release to suggest this is just minozidil. Why would they need to do clinical trials for that?
It says drugs that are already on the market (“on-market drug”). Combine that with the Minoxidil cartridges photo I discussed in the bottom half of the post. I would prefer them testing and using on-market topical Dutasteride :-)
What a Joke.
I’m echoing a sentiment expressed by Scott here when I say we should wait for pictures and not get so hyped by percentage points.
After all, a study using Topical Melatonin had a 41% increase in hair count. (Base Hair Count: 123/cm2; Month 3: 159/cm2; Month 6: 173/cm2). Another study was 42.7% (Month 0: 85.76 ± 27.0; Month 3: 110.82 ± 31.7; Month 6: 122.35 + 40.5).
A number count comparison would look like this:
Topical melatonin grew 50 hairs (41%)
Follica would grow 55 hairs (44%)
https://www.researchgate.net/publication/237844372_Topical_Melatonin_for_Treatment_of_Androgenetic_Alopecia
But what does it look like ???
Remember when you have 1 hair per cm2, just 1 new hair is a 100% increase. Some of us with long gone temples and foreheads and slick bald spots are gonna need a lot and a lot and a lot more percents per cm2 to get something cosmetically satisfying. : )
Is this treatment worth getting excited about? I think that depends on the answers to these questions:
1) What does 44% actually mean? Will that take a Norwood 3, say, to a Norwood 1? If someone has diffuse thinning that is visible, will this treatment reverse the thinning to barely noticeable? In short, will the difference be noticeable and visually significant?
2) How often do you need to do it? Will you have it done at a clinic that offers the service, then have to do it at home? Will you have to go to the clinic every few months? How long will the treatment “last”?
If “44%” is a visible, noticeable improvement, that has to be a game-changer. It has to.
If this treatment provides significant cosmetic improvement, and it “only” needs done once a year, I’d be delighted.
Guys I’m sure it’s a step up from regular minox and fin or they wouldnt do it…anonymous had a good point. I think they failed after all these years of coming up with another compound but they knew they had good old minox in their back pocket just in case. No way did it take 10 or so years to optimize a microneedler. Seriously, I and most of you could have done what they did. Just keep trialing different depths and amount of needles until I found which had the best effect. Now throw good Ol minox in there and tadaaa…people will buy….this scene sucks.
Ladies and gentlemen, I believe I found the study that Follica used as the main protocol of their invention. Basically, I believe they took this study and ran with it and tweaked it. Even their motorized needler/minox dispenser…i think this is huge and tells more about their process but I’ll let you be the judge. Believe it or not, in the study, one of the participants was using .5% minox. Now maybe all along when rogaine etc. Says 5% maybe it’s really .5%, I really dont know and maybe our pharmaceutical gurus on here can tell us? But seriously, let me know if you want the study as I rarely get a response on here so I’m not posting it unless I hear back….with all due respect.
Y u mad, bro?
It’s just microneedling and minox.
They tried other drugs out and they got no marketable benefit.
C’ est la vie.
Hopefully, Tsuji’s deed’s cost will be lowered at the 10,000-20,000$ level within the next ten years.
That’s our only hope and that has always been our only hope since 2016.
And by hope, I mean hope for a functional cure.
I’m pretty sure pharmaceutical choices will be available within the next five years, but with moderate effects.
Merry Christmas everybody!!!
Tomjones, I would like to see the study. And anecdotally, I bought an expensive micro needle pen several years ago, read all of the studies in detail and tried every combination I could think of over a year and a half time period with zero results. But I am starting up again based in this piece of information in the press release: “This process of hair follicle neogenesis involves minimal daily interruption”. So I am going to trial 1.5 depth once a week with quick daily disruption at .5mm. Perhaps follow up daily disruption is the key. I did read several studies several years ago that left open many questions about what happens when you micro needle but you could certainly interpret the studies to support the significance of daily disruption in keeping the “electrical” cross talk going as it apparently fades relatively quickly after disruption. Personally, I don’t think the detail with which they have honed the disruption process (depth, frequency, etc) is insignificant.
Their wounding technique/machine PLUS Samumed’s WNT hair growth drug = cure for me.
2021-2022 (at the latest)
If its just minoxidil, why dont they just release it now??
If it’s just minoxidil, then technically speaking it’s already out there :-) Knock yourselves out, but let us know if it works.
Hi Guys, my Dr prescribed 5mg of Fin and advised to take once a week, I took it for 2 mth and I stopped.
Fast forward one year later I’m thinking to get back on it, my question do most ppl use cutter to cut it to 4 quarter and take them daily? As that will be 1.25mg for daily intake will that be a lot for daily dosage?
I’m not using Minoxidil yet.
Appreciate your advise.
Hey Billa,
The 5mg Finasteride pill split into quarters and taken daily is commonly prescribed for hair loss. Yes it’s technically “1.25mg” in comparison to the 1mg name brand propecia Pill but there are other factors that could effect the quarter tablets “1.25”mg dosage. I have read before that the drug manufactures do not guarantee the 5 mg of active ingredients will be evenly distributed throughout each pill, meaning you could end up with certain pieces being above “1.25” and some being below “1.25”. Also consider splitting a pill into this many pieces almost never breaks into 4 small even pieces. When I was using generic 5mg Finasteride pills, most of the ones the pharmacy had in stock were not even round or evenly shaped pills. I had to find a specific manufacturer that still made a round 5mg pill and ask them to order it in specifically for me. The point is, it’s almost guaranteed you are going to be taking a fluctuating daily dose. Is this a big deal? I personally have no clue as I am not a Doctor or chemist, but I did not have good results from generic Finasteride. If I had a choice I would have simply take the name brand Propecia and not dealt with the hassle of the pill Splitting.
The obvious problem is the cost of Propecia is much higher than both Proscar or a generic 5mg Finasteride. Many insurance companies will still
cover both Proscar and generic 5mg Finasteride as they don’t consider this a hair loss drug as they do Propecia.
By the way, the 5mg Finasteride your doctor originally prescribed you to take once a week seems unusual. I wonder why he advised to take it just once a week?
I can comment on the 5mg proscar pill and why the active ingredients may not be evenly distributed when you quarter them. In my Pharmacy class, unless they are scored ( cut ), they most likely are not distributed evenly. Put it this way, they are not designed to be distributed evenly. That’s why say if xanax is scored and the Dr. writes….take 0.5 to 1 mg….That means the pill was designed to be distributed evenly. I also seen this during my clinical rotations in medical surgery. Not replacing medical advice, just educated guess. So in that regard, yes, Brand name Propecia is designed to be 1mg so thus it’s superior in my opinion ( I have never taken it myself ) in the fact all the active ingredients are consistent as opposed to splitting into quarters of fifths of a 5 mg procar tablet. Again, I have never taken Propecia…..thought about topical finasteride, but question that the generics probably used in a topical will change generic distributors all the time. If something works, you want it to be consistent sourced not matter generic or Brand name….hence why with brand names, you never have to worry, it’s always sourced from the same place/places. I am shocked most don’t know this. Pharmacies buy the cheapest they can get things, unless person tries to special request it and insurance covers. By the way, my last sentence is not to be insulting. Again, I am a Nw 2.5ish diffused and thought about finasteride etc. Never used.
PinotQ, do u have an email I can reach you at? I’d like to toss a couple things around and include the study. I will not be soliciting or anything weird, trust me.
Admin, Would you be able to send Tomjones my email address?
Hi Tomjones, please email me (contact page has my email) and I will then send you PinotQ’s email.
Thanks Admin!
Some of us respond very well to minoxidil, luckily I’m one of them. If I stop using finasteride for a few months, not much happens, but if I do the same thing with minoxidil it is a disaster :(
Which brand of mix you using? Does it grow your temples hair?
I use the 5% minox of the kirk … on the temples I had a minimum regrowth, for the most part vellus
Can any of the chem or science or smart people on here tell me that if a study says .5% minox, are they referring to the 5% version??? Thanks!
Most probably.
There is no .5% minox in the market.
I googled it and found out there’s a 0.5%/2mL regime.
Each can has 60mL, so the study probably used a 15%/60mL minoxidil solution.
Much stronger than the 5%.
0.5% means 0.5%, it’s unusual but from what I remember in the early days they tried different dosages up to 2%. Percentage is only one variable, you need to consider amount (ml) and frequency to get the actual daily quantity (mg). What’s the study about?
2019 Dhurat study on PRP having no clinical efficacy.
Mission Impossible: Dermal Delivery of Growth factors VIA Microneedling
“Several studies have demonstrated that for PRP to be efficacious, the therapy has to be delivered to the level of the follicular bulge, which lies at a depth of 1–1.8 mm below the skin surface, well below what the common microneedle can achieve. Thus, we suspected that most topical PRP microneedling procedures provide no clinical efficacy.”
https://www.researchgate.net/publication/332295122_Mission_Impossible_Dermal_Delivery_of_Growth_factors_VIA_Microneedling
Well at least they know why PRP is useless now. But some of us including myself knew this in 2015 when we were trying it.
Scott, To clarify, I don’t think that Dhurat study is saying PRP is useless. I read it to say that it is useless if applied topically by way of micro needling. I had PRP once and not sure how it was applied when I did it but in my experience, it was in fact useless. However, I have had PRP/Acell that was applied by way of injection to a greater depth followed by micro needling to supplement the treatment. It was effective and I sent Admin the comparative pics as evidence. Subsequent PRP/Acell treatments were not effective for me for some reason. My interpretation of this study is that micro needling isn’t going to improve the penetration of any topical that is greater in molecular weight than 500 daltons. I think this is useful to know if you are experimenting at home. For example, the molecular weight of minoxidil is 209. And Cortexolone, CB-03-01 is 402.
I thought it was interesting that a 1.5mm dermaroller only penetrated to a .5mm depth.
It depends on the application, Toccata.
If you roll pressing onto the scalp, you reach deeper.
There was a Greek guy on HLT who said that anything less than 1.5mm is worthless and all those idiots attacked him.
I’m the gay one and those dumbtards have gone completely feminized.
It was really sad to watch.
All those men… with no sense, at all.
Sad…
I should have mentioned what made it interesting was that it was Dhurat herself writing this. It is her study that is most leaned upon by the forum which has, as you say, insisted that anything under 1.5mm in depth is worthless. Most likely she never went much deeper than .5mm with her roller (until mild erythema, I believe she said).
In a few years we’ll know the Follica protocol. Especially if it is expensive, we’ll see members doing a homemade version with their own pens.
(I personally do a graduated approach: .5mm for a few minutes, then .75, 1. and finally 1.5. I run the machine on the fastest setting with small brush strokes like a tattooer)
I would go personally deeper at 2mm.
The Greek guy said that 0.5mm rolling only helps absorption.
So, it doesnt make sense to dermaroll progressively, I suppose.
You could dermaroll at a 1.5-2mm depth twice or three times a month, and at 0.5mm daily before applying minoxidil.
Thats what I understood the Greek guy actually recommended. He was constantly attacked by incels and idiots, so I got lost at some point…
I think he said he is a dermatologist, but I dont remember exactly. So, take that with a pinch of salt.
I don’t much pay attention to the HLT ‘absorption’ bit. Minoxidil instructions clearly state do not apply on skin that is red, painful, irritated, scraped, cut, or infected. A study in the Dhurat footnotes said you risk overdosing and experiencing cardiovascular side effects (and blurred vision, hypertension, fainting, irregular heartbeat). Dhurat’s own trial delayed using minoxidil until 24hrs after. Good for me.
There is a study on the optimal depth being .25mm / .5mm —not higher. The conclusion: “Our study provides evidence that microneedle stimulation can induce hair growth via activation of the Wnt/β-catenin pathway and VEGF.” (But it’s mice)
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5064188/
If you’ve needled you’ll know a pen at 1.5mm and above will make you bleed instantly. The blood makes the areas you need to cover more obscure and difficult to treat—and painful! An important component of this procedure is needle density—perhaps even more than depth (Follica writes about this quorum sensing study in their booklet). So I start at a lower depth and go over my head more meticulously. By the time I draw blood I have needled well all the areas.
“the collective hair follicle response to injury may be seen as an example of quorum sensing, a form of social behavior in which population decisions depend on the density of signaling individuals within a given spatial territory” https://www.sciencedirect.com/science/article/pii/S0092867415001828
Anyways, Merry Christmas and Happy Hairgrowth!
~I’ll see y’all again in *duh-duh-duh* 2020!~
That Greek guy is an imbecile. Toccata has the right idea. Follica’s own documents give a depth of ~0.15mm. It’s all about the density of the wound, not the depth.
Guys that study is saying topical prp with microneedling. That is far different then regular prp INJECTIONS. Prp works for many, you just have to keep going back every 2 months which most cant afford…..now getting back to the .5% minox. I found a study where it is effective! I have a suspicion that follica is doing similiar.
I am planning to start PRP. Why 2 months? Any sources? From the studies I read it seems that maintenance can be achieved with a treatment every 6-9 months. I am not sure why they recommend a higher frequency in the first few months though.
Hi Admin, thanks for the update and for all of your thoughtful posts.
I’ve been wondering something about the whole “wounding plus minoxidil” thing; specifically, do the studies on wounding plus minoxidil include ongoing minoxidil use (with the direct hair growth benefits that that entails), or is there really a special effect of minoxidil applied alongside the wounding process that is independent of whether one continues to apply minoxidil twice a day? In other words, does one have to keep applying minoxidil on an ongoing basis for the potential wounding gains to be effected and preserved?
Any Minoxidil related treatment needs permanent usage. Perhaps the wounding can grow some hair that never requires Minoxidil once generated. But no studies on that as yet.
I tested the reverse of this a few years ago.
I used a Derminator with 12-pin 1.5mm needles every Sunday and applied Kirkland Minoxidil 2x Mon-Sat.
I did this for 6 months. I had either new hair growth or existing hair thickening (I can’t say). Either way, I could confirm absolutely an improvement visibly—though, it wasn’t miraculous, I wouldn’t say I recovered a full Norwood.
I stopped microneedling but continued applying Minoxidil 2X daily. I hoped I would maintain the current density level and build on it the following year when I went back to needling.
NOPE! For whatever reason I fell back into baseline, now I’m a little worse, despite continuing minoxidil this entire time.
I’m going to add Finasteride in a few months (*sigh*) and Microneedle + Minoxidil through the whole 2020 year. If Tsuji and Shi/Replicel and Rapunzel, Tissuse, etc are still silent by 2021 and things are still up in the air, I’ll probably get a hair transplant (*double sigh*)—I live near Dr. Rahal so it is convenient—and Toppiks the rest. Done.
a couple years ago I was stressing and I lost a chunk of my hairline. I dermarolled once a week 1.5mm along with minox and the hair came back although i didn’t get much of any regrowth in my crown. I quit using minox in that area once it grew back and its still intact till this day.
“The study involved a less than five-minute in-office experimental scalp procedure…” — “This process of hair follicle neogenesis involves minimal daily interruption”
Unless they plan to sell the HFN device commercially, going to a dermatologist daily, even for 5 minutes, isn’t really viable for most people. They’ve advertised this as being an in-office procedure, but it doesn’t look feasible.
Yeah…you are completely misunderstanding what they mean.
I think I want to give microneedling a chance. What should I try dermaroller or dermapen?
pay attention man
Derminator 2 and a year supply of 12-needle cartridges. B^)
Adding to the discussion on micro needling and depth vs frequency, see https://elifesciences.org/articles/28875 and https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3921236/. Much of this technically over my head but there are discussions about wounds at .5 mm closing quickly by electrical stimulation (safe no scarring) and wounds up to 1.5 mm initiating various phases of healing lasting months (presumably no scarring if not repeated too frequently). There is also a discussion of cross talk between cells near the surface and hair follicle cells further below. And that only a small number of cells near a damaged site will respond. Crudely summarizing here but you could interpret this to mean you need 1.5 mm type wound coupled with daily minimal (.5 mm) wounding to keep the cross talk going. Obviously many other factors and nuances involved but this interpretation gets you closer to the clues being left by Follica’s latest press releases of a deeper in office disruption followed by daily minimal disruption.
in-office disruption, at-home *interruption*
Daily minimal interruption means it will hardly interfere with or burden you in your day-to-day life. They are talking about your state-of-mind applying the topical using their dispenser and tracking your progress with their phone app (which you can apparently use to re-order the topical) and not finding it an inconvenience. : )
It’s not just 5 minutes in the office…it’s the hassle of scheduling and driving to and from the office. The question is how often it has to be done. Once a month? Quarterly? And…for the rest of your life, presumably, or at least until something better comes along.
Hopefully SM and Breezula get me over the top. I don’t think Follica is going to be all it’s cracked up to be when you consider all the factors involved. If it’s quarterly office visits and its affordable, I’m all in. But I would guess it’s much more office visits and not terribly affordable.
These results are after a single treatment. You’d only need to go 2-3 times total.
You just have to keep following the at-home regiment.
So much hype for nothing … Follica is synonymous for joke …
But don’t worry guys 2020 is here and so is our miraculous cure… right? Well sooner or later it has to come, at this point I don’t care if I can benefit from it or not… I just want it to come out because baldness simply SUCKS.
The biggest joke is actually people like yourself.
Please go ahead and tell us what else can reliably grow 44% more hair after a single use, with no scarring or astronomical cost?
I’ll spoil it: Nothing. Not DIY dermarolling. Not transplants, not minoxidil alone. Nothing.
After 2-3 sessions of this, you’d be pretty much back to normal.
Yet, you’re still not satisfied because it doesn’t meet some stupid sci-fi perception of a “cure” that you have.
THAT is the joke.
But, absolutely nothing grows hair back RELIABLY. There are countless studies on everything from minoxidil to nutraceuticals to microneedling/prp and even laser light therapy that tout 50% improvement. And as always the studies focus on particular patients with a thinning crown and high degree of follicular activity in the region( ie..the follicles weren’t truly dormant and signaling was still active). Follica falls into the same category as other microneedling/minox/prp/growth factor products.
Positive, but underwhelming. I pity people that are sitting around thinking “yeah I’m gonna regrow my bald temples with Follica.”
DIY microneedling and Follica are far more similar than you want to admit. The difference is that Follica is trying to monetize something that is already available by making it as proprietary as possible.. “you’ll need our special minoxidil which you can order by our handy app.”
John Cole was part of a study that found 90+ hairs per cm2(!!!) using activated prp.
“Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems”
Hell, everyone should just get activated PRP and move on with their lives. Unless, it only grows substantial hair in certain highly selected patients in a particular region of a thinning crown or something…..
Look at the minox studies. The early studies claimed things like +40 hairs per cm2, but a recent study found less than 1 hair per cm2!
TLDR:
Follica will give similar to results to other wounding/prp/needling/adipose/amniofix/growth factor etc treatments. They are trying to monetize Dhurat’s simple study. Nothing wrong with that, but you are crazy if you are thinking this some next gen tech.
Some derms will add this to their list of tech treatments. It will fit in nicely next to the laser helmets.
I guess you don’t know much about Follica…but it’s sweet the way you hope for your 44%…LOL
If you got 40hf/cm², microneedling and minoxidil just adds 17hf/cm², so 57hf/cm².
Yep, that’s the cure right there.
Man, you are unbearably stupid.
You always talk BS.
I still remember when you would insist that if Riken says 2020, it will be 2020.
DUMB is forever.
I know that admin will sometimes post info on grey hair. I’m about 75% grey now – at least I still have some hair on top, but I look much older than my age just because of my grey. I’ve been looking for a solution for years now and I don’t see anything that’s even in the research pipeline for grey hair (L’oreal’s 2006 media b.s. pill doesn’t count).
I have been looking for a temporary color solution that doesn’t look fake. I tried Just for Men, etc., years ago – nothing like that impressed me. Just looks fake on men.
I’ve been keeping my eye on this product that came out this year:
https://amzn.to/2Zi63nY
I’ve been skeptical of it from day one, but fakespot.com still gives the product reviewers an “A” grade, meaning there isn’t too many fake reviews. I still won’t buy it until I see many more reviews of it. For anyone else out there who is looking for a temporary grey solution, you might keep an eye on this. For now, I remain skeptical. The price is a little steep to get ripped off for me. If anyone tries it and has success (or failure) with it, maybe you can mention it . If the reviews are still good months from now, I’ll try it.
I’ve just started using the shampoo Plantur 39 to help hide some grey hairs. It’s inexpensive and apparently it’s efficacy builds over time.
This hair loss article about DSC’s appeared on Google’s front page:
https://www.google.com/amp/s/medicalxpress.com/news/2019-12-hair-growth-baldness-optional.amp
Thoughts?
Interesting, it could lead to the first non-hormonal maintenance treatment.
Good to see potential new treatments on the horizon but what I got from that article was trying to figure out how to stop balding from the beginning stages – It’s not going to regrow hair in bald or thinning areas.
How may times have we read a headline in the news with a new break through or discovery in the hair loss community for a new treatment or cure involving mice lol.
How insane would it be if Dr. Takashi Tsuji came out next year with the cure for hair loss. Even though I can’t afford it, just knowing something out there actually works.
Once you stop the root cause, you can use neogenesis techniques like wounding and minoxidil (plus whatever else Follica has up their sleeves) to regrow it.
The main thing I felt from this article is that the hair loss cure industry is picking up – I didn’t find the article itself that fascinating but what I found exciting is that this is being talked about a lot more in the news and media. I think people care a lot more about how they look today so hair loss, something you would just have to deal with regularly, is now being looked at as a solvable problem by a bigger audience – like everyone who sees Google’s homepage. That’s what I find exciting. You guys?
I agree with that guy about not a sci fi cure. If it’s existing topical I don’t care. Does it regrow thick dense hair is what matters. However, 44% regrowth based on their picture doesn’t really beat hair transplant results. The guy still has a bald spot. It’s less noticeable but he is still balding. The treatment obviously works but we were all hoping for something much better based on all the hype. I used to be a big follica fan, still am but disappointed in that picture. Hopefully that was just a marketing gimmick and that they release pics from the recent results and those show amazing regrowth. We deserve it and it’s been 20 years since a hair loss treatment came out. SM will only give 11% based on their phase 2. So follica will be the better treatment. Going to a derm for the in office procedure once or twice a year or even quarterly isn’t too bad if it can grow real coverage. Beats getting my scalp cut, not sleeping for a week, and embarrassment of walking around post fue with bloody scalp. I still have faith in follica, even though I am kind of disappointed in that pic. We shall see what happens in 2020. I would have been ecstatic to see that bald crown filled up with terminal hair.
Michael Rendl, MD
There is no clinical trial. We just discovered the fascinating mechanism by the sheath during catagen regression in mice. And now we begin to test in tissue culture the effects with human hairs which will take time. There is a long way to go before things can be tried out with people. I hope you understand!
I appreciate your enthusiasm and willingness to help with a clinic trial.
“Drugmakers Turn to Data Mining to Avoid Expensive, Lengthy Drug Trials” – Wall Street Journal
https://www.wsj.com/articles/drugmakers-turn-to-data-mining-to-avoid-expensive-lengthy-drug-trials-11577097000
Interesting read on accelerated drug approvals for medications that are already FDA approved and are being used for off-label use. Maybe this can help us get hair loss drug approvals faster.
Also, speaking of drug availability and access, maybe finasteride, or at least topical, will go over-the-counter in the near future. Especially since the FDA is considering moving oseltamiver (Tamiflu®), for influenza virus, to over-the-counter. https://www.pharmacytimes.com/publications/issue/2019/december2019/tamiflu-set-to-switch-to-otc-status
– Phil Collins, PharmD
@x3442 where did you hear about Dr Cole being part of a prp study that grew 90 hairs cm2? That right there is a functional cure. Thick coverage and great! Is this real or bs? I’m leaning towards bs because that would have been all over the forums and news.
Mjones,
It was:
“Evaluation of Not-Activated and Activated PRP in Hair Loss Treatment: Role of Growth Factor and Cytokine Concentrations Obtained by Different Collection Systems”
Yet, at the same time Cole was on the Baldtruth talk show recently talking how neither PRP nor exosomes would would work for follicles that had lost their ability to signal.
Nobody should take simple figures like +x hairs or +x% of hair seriously.
If you had one hair on your crown and doubled it that’s a 100% gain. Likewise if you have ~50% of your hair in a region it’s common to get extensive regrowth, but you have extensive loss in a region regrowth is very unreliable.
I’ve had PRP and I’ve benefited from it but it’s no miracle and it’s too expensive for me to recommend for the average person.
Follica could eventually become something. Perhaps a future version will have the necessary components to be game changing.
But, at this point I see everything including microneedling+ minox as unreliable and only worthwhile as part of a long term combination approach.
Wise man, I salute you!
Could this blocking muscular contraction be why Botox is touted to help with hair loss?
Exactly what I thought :-) Though no miracle hair growth reports on the Botox as yet.
Admin do know what the status is of:
Poietis & L’Oreal: Bioprinted Hair Follicle ?
Hi pjotre, have not followed them recently, but will try to in 2020 :-) They never show up in my general hair loss Google Alerts.
FINALLY! :)))))))
Hey Admin, thanks for the update on Follica.
Something really new and highly interesting, if not a sensation:
While Exicure is doing research behind closed doors with major funding, South Korea university of Ajou published the first study of CRISPR/Cas-9 using to treat androgenetic alopecia. From what I understand, this looks very promising already in this early stage.
https://www.crispr101.co.uk/post/crispr-restores-hair-growth
https://www.sciencedirect.com/science/article/pii/S0142961219308543
Ultimately this will be the therapy which will make hairloss not just optional, but also very easy to treat and affordable for everybody.
What’s your verdict, admin?
Thanks Ben. I used to cover CRISPR in my “brief items of interest” posts, but should write another post on this soon. Edit: I covered this study in January:
https://www.hairlosscure2020.com/dermal-sheath-contraction-lymphatic-system-and-crispr/
any idea when follica could potientialy be released?
https://www.nature.com/articles/s41586-020-2352-3
Hair-bearing human skin generated entirely from pluripotent stem cells
https://www.nature.com/articles/d41586-020-01568-2
Regenerative medicine could pave the way to treating baldness
Great update.. Follica looks to have serious potential. Not sure why I wasn’t more excited with December announcement. A 44% increase in visible hair count is fantastic. The next trial is proof that things are actually moving forward, so let’s hope for some swift progress :-)
Just making sure I understand this correctly – this is saying that the people who used Follica had their amount of hair increase by 44%??????
Hey admin, any idea for a release date ? Can we expect it H2 2021 / Early 2022 ?
It’s been a while since Follica has toyed with my emotions. I look forward to seeing what they have in store for me tomorrow. (Or is this the moment that I can finally break free of them for good ???)
Unfortunately, no product updates in the Annual Report….but curiously the Follica webpage is currently down. My hopes for them toying with my emotions have not been dashed yet! :)
Thanks! Have not followed Follica or Puretech for a while.
I feel like Follica is playing with us..i had such high hopes. I remember in 2007 i saw a TV interview with a Follica exec saying it will hit market by 2011 because they were using things that wouldnt take long for FDA approval. Here we are 16yrs later and most of the news ive heard over the years was only about how much money they keep raising. Tens of millions of dollars and almost 2 decades later and all they have is a micro abrasion device and Minoxidil???? And once again no news or updates for a while… i think these people are just making money from the fund raising….