In just the past month, I covered two different companies working on a latanoprost based hair loss treatment (Canada-based Triple Hair and US-based Dermaliq). Both companies are almost certain to continue to Phase 3 clinical trials in the near future.
I also covered US-based Aneira Pharma and its latanoprost containing combination hair loss product in 2021. While the company has not updated its website for a few years, its patent application status page has been updated a few times in 2024.
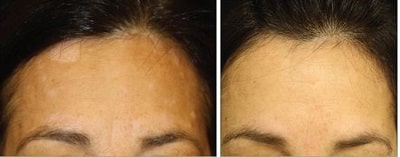
Latanoprost is a prostaglandin F2α analogue eye medication that is meant to be used to treat glaucoma and ocular hypertension. It was approved for medical use in the US in 1996. An unusual side effect of latanoprost is increased and thicker eyelash hair growth. A rarer side effects is eyelash hair darkening (see image above from here).
BioResearch Pharma BRP-011: Latanoprost Acid for Hair Growth
Now comes news of BioResearch Pharma (Poland) working on a latanoprost acid based hair loss product called BRP-011. Thanks to “Ben” for posting a link to a very encouraging recent Polish magazine interview with CEO Lukasz Zybaczyński. This company was originally mentioned by “John Doe” in a comment earlier this year, and he also e-mailed me the above interview link.
According to the BRP-011 product page:
- Latanoprost’s high skin penetration rate has raised concerns about potential systemic adverse side effects.
- Consequently, latanoprost acid (an active metabolite of latanoprost) has emerged as an alternative option.
- Latanoprost acid was initially dismissed as a potential topical treatment due to its low bioavailability.
- However, BioResearch Pharma (BRP)’s chief scientific officer and co-founder Dr. Katarzyna Koziak has shown latanoprost acid to promote hair growth in a clinical setting involving indiviuals with androgenetic alopecia.
Note that BRP is only focusing on treating two conditions: androgenetic alopecia and psoriasis (via drug candidate BRP-007).
Commercialization in 2027
In the new CEO interview, the most interesting points relate to a very optimistic timeline forecast due to the nature of BRP’s products. Key quotes in bullet points (wording slightly modified by me):
- BioResearch Pharma does not want to discover a new medicine and prove its safety, which usually takes years. The company intend to use an existing active substance in a new mechanism of action in a new route of administration. Regulatory agencies allow the registration of a medicine in a new therapeutic indication, using publications and data that are not the property of the applicant.
- In the case of a new chemical molecule, the initial stages of the drug development process related to production and pre-clinical evaluation take around 5 to 7 years. In BRP’s repositioning process, they shorten this period to a year, relying on the past experiences of other pharmaceutical companies and patients. This method confirms safety standards.
- In total, an up to 9 year process will be shortened to 2-3 years by BRP. i.e., much faster clinical trials.
- In 2025, the company will begin the registration process.
- The CEO anticipates commercialization after completing Phase I of clinical trials. This should happen by the end of 2026 for both projects (androgenetic alopecia and psoriasis). So commercialization in 2027.
The latter part of the interview relates to fundraising and a pending IPO.