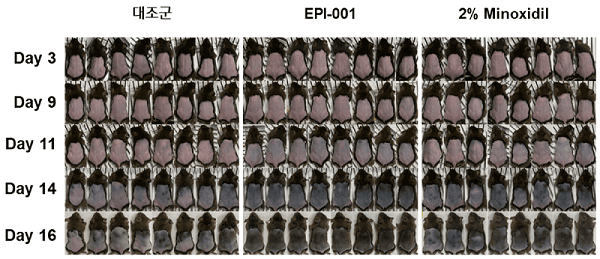
Recently, Yokohama National University (Japan)’s Dr. Tatsuto Kageyama published a very lengthy and detailed paper in a Japanese scientific journal called Journal of the Society of Bioengineering. Dr. Kageyama works at the renowned Fukuda Lab in Japan, which is led by Dr. Junji Fukuda. The actual paper is in Japanese and is titled: “Bioengineering approaches for hair follicle regeneration.”
I have translated it and will break out the main details in this post. Besides discussing his team’s scientific approach to hair regrowth, Mr. Kageyama also outlines their overall progress and future plans (though he gives on exact dates for clinical trials). Make sure to check out the diverse range of Google Scholar citations for Mr. Kageyama. In most of his important hair growth related papers, he is a co-author with Dr. Fukuda and others.
Previously, I also mentioned that Ayaka Nanmo, Tatsuto Kageyama and Junji Fukuda are co-founders of a new company named TrichoSeeds. It aims to provide “hair regeneration medicine.”
Bioengineering Approaches for Hair Regeneration
To start off, Mr. Kageyama outlines the three main methods of hair regeneration that his team at Fukuda Lab is working on:
- Mesenchymal cell transplantation. Reactivates existing weakened hair follicles by injecting mesenchymal stem cells.
- Hair follicle primordium transplantion. See their pending patent on hair follicle primordia.
- Hair follicle regeneration. The creation of brand new hair follicles in those who have none left. See their January 2024 study on the large-scale preparation of hair follicle germs (HFGs). The transplantation of HFGs in mice resulted in highly efficient “de novo” hair follicle regeneration.
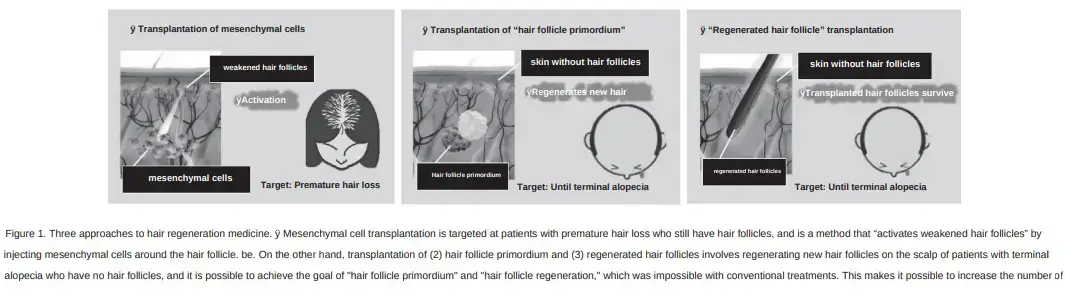
I am most excited about the first method for now since it will come to market first. Especially in aging Japan, where autologous regenerative medicine treatments are going to be speed tracked to in-clinic use. In fact in my recent post titled “A Visit to Fukuda Lab“, Dr. Junji Fukuda had the following quote:
“Dermal papilla cell transplantation is about to begin in Japan.“
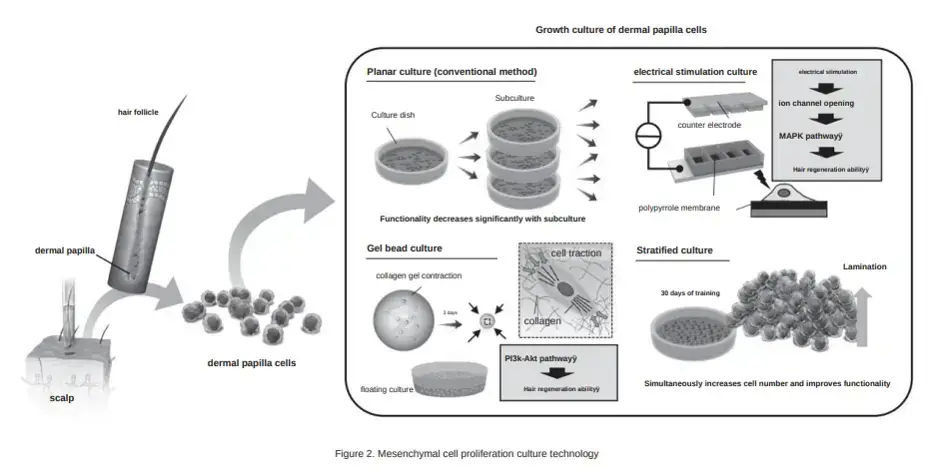
If you have a reasonable amount of your own scalp hair remaining, this mesenchymal stem cell injection treatment sounds very promising. The diagram below that Mr. Kageyma published in his new paper is extremely detailed.
Techniques for Culturing Dermal Papilla Cells
Mr. Kageyama discusses his team’s three unique approaches to culturing dermal papilla cells. I covered these methods in various posts and updates related to Dr. Junji Fukuda’s published papers over the past decade.
The conventional dermal papilla culturing method (number 1 below) is similar to Shiseido’s work, which Mr. Kageyama discusses briefly. He says that it has limited effectiveness, since the hair regeneration ability of the cells is reduced during the process of growing mesenchymal cells in a culture dish.
- Conventional culturing.
- Gel bead culture.
- Electrical stimulation culture.
- Startified culture.
Hair Regeneration by Transplantation of Hair Follicle Primordia
In this second key approach, the aim is to increase the total number of existing hairs. Dr. Kageyama also discusses the work of Dr. Takashi Tsuji and his team in this section of the paper.
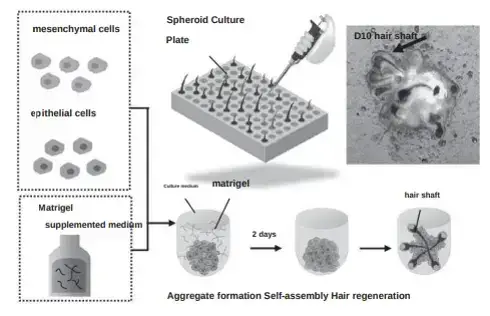
The gist of this technology is the mixing of epithelial cells and mesenchymal cells into a single aggregate. This in turn results in the formation of hair follicle primordia (hair seeds), which can then be transplanted into thinning areas of the scalp. The Fukuda team has already succeeeded in the large-scale preparation of hair follicle
primordia.
At present, the team is conducting experiments in transplanting human hair follicle primordia into mice and improving the regeneration efficiency. At present, there is no guarantee that hair follicle will regenerate from the transplanted seeds. It is also difficult to control the direction of new hair growth with this approach.
Hair Follicle Regeneration
The third and final approach entails the creation of brand new hair follicles for transplantation. i.e., hair multiplication. The Fukuda Lab team has managed to develop a culturing technique for regenerating hair follicles in vitro. The discussion here is fairly technical, so I will just quote (and hope that the translation is accurate):
“Focusing on the self-organization process of epithelial cells and mesenchymal cells, we regenerate mature hair follicles with high efficiency (>99%) by controlling the spatial arrangement pattern of aggregates that form in the early stage of culture. Controlling the spatial arrangement of epithelial cells and mesenchymal cells in the aggregate on the second day of culture from a dumbbell shape to a core-shell shape was the key to in vitro hair regeneration. The production method is very simple, such as adding approximately 2% Matrigel to the culture medium when seeding epithelial cells and mesenchymal cells.”
Future Plans
The team plans to continue its work in all three approaches to hair regeneration. i.e., transplantation of mesenchymal cells,
transplantation of hair follicle primordia, and transplantation of regenerated hair follicles. Moreover, they have succeeded in constructing microtweezers that can grasp, cryopreserve, and eject hair follicle primordia. They are also working with robotics experts to develop a fully automated transplantation robot.