Update: February 29, 2020
Aclaris Exits Hair Loss Business
Recent rumors are, unfortunately, very true. Several days ago, I contacted Aclaris Therapeutics’ Head of Corporate Strategy Mr. Michael Tung. I asked him for any update on their androgenetic alopecia (AGA) JAK inhibitor product. His short and succinct response was as follows:
“We exited all of that unfort”.
I am disappointed, but was expecting this bad news. Their pipeline page no longer even shows any products to target AGA or Alopecia Areata (AA). Last year, they were searching for a partner to help further develop their “investigational compounds” ATI-501 (oral) and ATI-502 (topical).
Sorry to reader “nasa_rs”. However, not a big surprise after the past year of limited information releases by Aclaris regarding their alopecia products.
March 18, 2019
Aclaris JAK Inhibitor Trial Update
I used to cover Aclaris Therapeutics and its topical JAK inhibitor product for androgenetic alopecia fairly regularly. However, there have been very few significant recent updates.
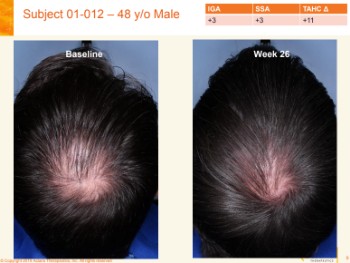
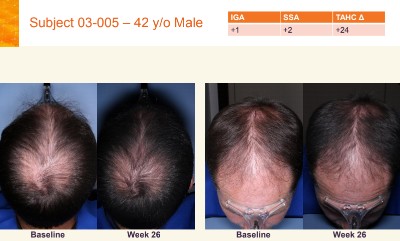
Today morning, Aclaris released a summary of its FY 2018 results. The most relevant part for us concerns the ATI-502 topical Janus Kinase (JAK) 1/3 inhibitor product for AGA. They also seem to name this same product as AGA-201 and ATI-50002 in other references.
Per the latest news, Phase 2 open-label clinical trials for ATI-502 (a topical JAK1/3 inhibitor) involve 31 patients with AGA. Interim 6-month data are expected in the second quarter of 2019, while comprehensive 12-month data will be realized in the second half of 2019.
So by the end of this year, we should finally have at least some idea on whether JAK inhibitors can at least somewhat help patients with AGA (aka male pattern hair loss).
The trials will measure both safety and efficacy.
We already know beyond a doubt that JAK inhibitors significantly help people with the less common alopecia areata. As well as thos with its various related forms (alopecia totalis and alopecia universalis).