I first covered RiverTown Therapeutics and its RT1640 hair loss product briefly in June 2016. My opinion of this company at the time can only be described as being highly skeptical.
RiverTown Therapeutics RT1640
This changed slightly in December 2016, when I wrote a post on Cyclosporine and hair growth. In that post, I discussed RiverTown’s RT1640 in much more detail. This product consists of three small molecule drugs that each work via different pathways and mechanisms:
- Cyclosporine A — FDA approved and off-patent.
- Minoxidil — FDA approved and off-patent.
- RT175 — A proprietary new chemical entity. Still needs FDA approval.
The final topical product will therefore entail synergistically attacking hair loss via three distinct mechanisms. According to RiverTown:
“One drug promotes the growth and migration of new stem cells, one drug promotes the commitment of the follicular stem cells to become new hair and one protects the dermal papilla from the effects of DHT.”
Note that RT1640 is equally effective in men and women. RiverTown’s science page has some other items of interest.
Dr. David Weinstein
Last month, The New Yorker magazine ran a cover story about the pending arrival of a cure for hair loss. To my huge surprise, they gave the most amount of coverage to RiverTown Therapeutics and its co-founder Dr. David Weinstein. Of the large number of companies working on a cure for hair loss, I would definitely not put RiverTown in my top 5. Nevertheless, I now do take this company much more seriously than I did in the past.
Moreover, RiverTown is one of the main companies that will be featured in a soon-to-publish article on future hair loss treatments from NewBeauty magazine. In that article, Dr. Weinstein claims that he is hopeful that RT1640 will be available in 2022 via prescription. Both as a topical lotion scalp hair loss product, and a cream eyebrow hair loss product.
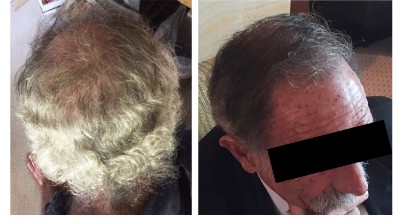
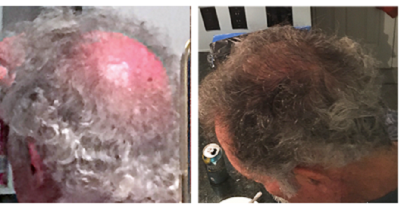
I therefore finally contacted Dr. Weinstein about his RT1640 product. He was more than happy to respond to me, and sent the below photos of his own before and after RT1640 scalp hair. The second photo was in a pdf document and lost some quality after extraction. I may try to get a better version.
Dr. Weinstein told me that:
- They are currently seeking funding to move into Phase 2 trials.
- He is convinced that RT1640 grows back lost hairs in their original pigmented format. i.e., the drug can reverse achromotrichia.
- The proprietary molecule RT175 has been tested in 650 patients to date, with no safety issues.