Mallia Therapeutics (formerly MalliaBioTech) is a Germany-based company that is developing a novel hair loss treatment based on a soluble version of the CD83 protein (i.e., sCD83). The bottom of this post is my original 2021 write-up about this company, with new updates on top.
Update: September 15, 2025
Recently, someone from a company that handles Mallia Therapeutics’ communications e-mailed me the below update:
“I wanted to share a quick but substantial update on Mallia, which you last covered back in May. Since then, Mallia has undergone a strategic reorganization and is now operating as a holding company, Mallia Innovations GmbH, with two subsidiaries focusing on therapeutic and cosmetic applications of its sCD83-based technology platform.
The therapeutics arm, Mallia Therapeutics GmbH, continues to advance MAL-856, a drug candidate based on sCD83, aimed at treating various forms of alopecia as well as wound healing. The company is planning to advance the therapeutic candidate toward clinical trials.
At the same time, Mallia has launched a second subsidiary, Mallia Aesthetics GmbH, to develop innovative cosmetic applications for hair growth based on a related molecule, MAL-838, which is also derived from sCD83. Their focus is on establishing a line of hormone-free, science-backed cosmetic products under the name 8T3 that are based on the mode of action of sCD83. The launch of the first 8T3 products is planned for 2025, with the goal of making advanced, research-driven solutions accessible to both professionals and consumers.
To support the build-out of both subsidiaries, Mallia recently completed a 5.5-million-euro seed financing round. The capital will primarily be used to bring the cosmetic product line to market while continuing development work on the therapeutic candidate.
The company has also formalized two advisory bodies. A corporate Advisory Board now includes Jens Holstein (former CFO of BioNTech), Dr. Alexandra Ogilvie (dermatologist and digital opinion leader), and Dr. Ulrich Dauer (serial biotech CEO). In parallel, a Scientific Advisory Board has been established, featuring leading experts in dermatology, translational research, and hair biology, including Prof. Dr. Ulrike Blume-Peytavi, Dr. Claire A. Higgins, Dr. Gillian Westgate, Dr. Geert Cauwenbergh, and Prof. Dr. Franklin Kiesewetter. These groups will provide strategic and scientific guidance as Mallia advances both clinical and consumer-facing programs.
With the new structure, Mallia wants to deliver on its original therapeutic vision while expanding into the cosmetics space for rapid market entry.”
Update: May 28, 2025
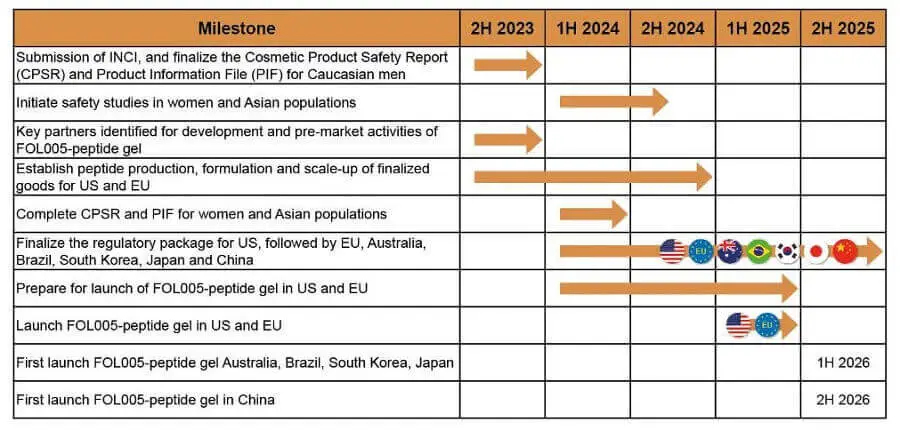
Mallia Therapeutics: 8T3 for Hair Regeneration
It seems like Mallia will soon release two cosmetic hair loss products that are based on the ingredient MAL-838 (a formulation of the soluble CD83 protein):
- 8T3 Essentials for at-home use.
- 8T3 ProLine for profession use.
Note that they also have a page on MAL-856, which is described as “a structural variant of the extracellular domain of the membrane bound form of CD83 (mCD83).” Both alopecia areata and androgentic alopecia are mentioned on that page. It is unclear whether the company still plans to conduct clinical trials and release a stronger non-cosmetic product in the future.
- Mallia Therapeutics will participate at the 2025 annual meeting of the European Hair Research Society (EHRS) in Warsaw, Poland from May 29 to 31, 2025. Their presentation is titled “Soluble CD83 as a novel therapeutic approach for the treatment of alopecia.”
- Also of note, in February 2025, Mallia Therapeutics and Northway Biotech (Lithuania) announced a partnership for the development of the production process and manufacturing of Mallia’s soluble CD83 protein (sCD83). Northway is a biologics Contract Development and Manufacturing Organization (CDMO) with facilities in the US and Lithuania.
Update: June 18, 2024
Mallia Therapeutics expects to start 150-person clinical trials at the beginning of 2025.
Update: July 11, 2023
Mallia Therapeutics Secures Seed Funding
Mallia Therapeutics (previously MalliaBioTech) finally has a website. Moreover, the company just announced that it has secured seed funding. They now plan to raise Series A funding prior to beginning clinical trials.
The company is developing a soluble CD83 (sCD83) based topical treatment of hair loss.
This sCD83 has an immune-modulatory function and induces hair growth via a dual mode of action:
- It induces an anti-inflammatory environment around the hair follicles via regulatory T cells (Tregs). These interact with follicular stem cells and activate hair growth.
- It directly binds to follicular stem cells and induces the formation of new hair follicles. So sCD83 not only prevents hair loss and accelerates growth, but also induces the growth of new hair.
Moreover, this treatment will supposedly work for both androgenetic alopecia (AGA) and the less common alopecia areata (AA).
Key quote (slightly reworded):
“The company is led by world leading experts in the field of CD83, with more than 60 CD83-related publications and 20 years of relevant experience.”
November 4, 2021
A new biotech company in Germany named MalliaBioTech is working on a topical hair loss treatment based on the CD83 molecule.
Mallia Biotech, CD83 and Hair Loss
On October 27, the Friedrich-Alexander University (FAU) in Germany published an important page on their site titled:
“FAU project wins funding for remedies against hormone-related hair loss.”
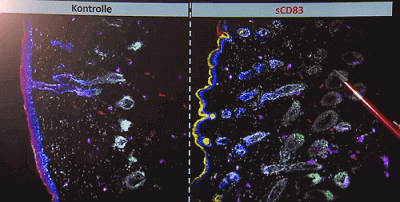
An soluble CD83 based active ingredient newly developed at FAU leads to new hair growth. The FAU researchers from the Department of Immunomodulation and the Department of Dermatology at the University Hospital Erlangen received the m4 Award for this project on October 21, 2021.
So far, this CD83 (see gene card) based active ingredient has not shown any side effects in pre-clinical studies.
The research team is led by Dr. Alexander Steinkasserer, who I e-mailed for more information. His co-researchers include Dr. Dmytro Royzman and Prof. Dr. Carola Berking. Their new company is called MalliaBioTech, and it received EUR 500,000 via the m4 Award. It is officially called the m4 Award from the state of Bavaria.
“The new product has the potential to conquer the large unfulfilled market of hair loss.”
In contrast to existing hair loss treatments Finasteride and Minoxidil, this topical treatment based on a soluble form of the CD83 molecule:
“Stimulated the formation of new hair follicles and thus induces new hair growth.”
While the current pre-clinical work is in mice and yet to enter human clinical trials, this research represents yet one more new method to tackle androgenetic alopecia. We read about at least five such major new hair loss cure related discoveries every year, so reader skepticism is warranted.
When I first heard about CD83 for hair growth, I assumed it would be for alopecia areata. This is due to the frequent use of the word “immune” in tandem with CD83. However, the new German research clearly indicates that this treatment will also work for hormonal hair loss (aka androgenetic alopecia).