
Veradermics (US) is working on a new extended-release oral Minoxidil tablet to treat androgenetic alopecia (AGA). The product is called VDPHL (as well as VDPHL01) and is currently in Phase 3 clinical trials. The top half of this post contains new updates in reverse chronological order. Also see my past posts on immediate-release low-dose oral Minoxidil and sublingual Minoxidil.
Update: October 16, 2025
Veradermics Raises $150 million in Series C Financing
Yesterday, we heard that Pelage Pharmaceuticals (US) raised a massive $120 million in Series B Financing. In 2026, they plan to begin Phase 3 trials for their hair growth product PP405.
Today, we have news that Veradermics has raised an even larger $150 million in an oversubscribed Series C Financing. And this is after they raised $75 million in 2024 from Series B Financing. Such numbers are unheard of in the hair loss world. Veradermics is already conducting multiple Phase 3 trials for their hair growth product VDPHL.
For context, in 2021, the esteemed RIKEN and Dr. Takashi Tsuji of Japan were finding it difficult to raise $5 million and even asked me to help them with the goal. And in 2025, Yunce Biotech (China) is finding it tough to raise just $2 million to move forward with their hair cloning work. See the most recent comments from reader “Jan Miedza” in that Yunce post. And in late 2024, the much hyped Stemson Therapeutics (US) folded because it could not raise sufficient funds. Many other hair loss companies that I have discussed on this site in the past folded due to lack of funds to proceed with tedious clinical trials and approval processes.
In the earlier mentioned press release from Veradermics, they do not mention any other ingredient besides extended-release oral Minoxidil in this VDPHL01 tablet. The do provide some great before and after hair growth photos from their Phase 2 trials:
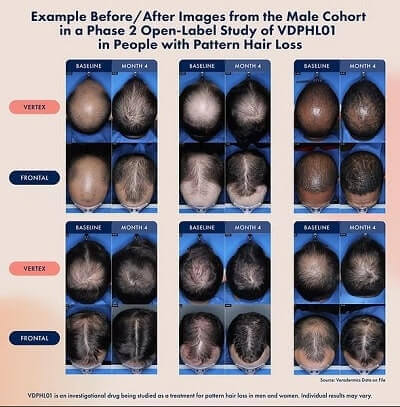
VDPHL Clinical Trial Updated Links
Enrollment links:
- 70 patients (male and female).
- Start date = 2024-07-08.
- Completion date =2026-08-28.
- 480 patients (males only).
- Start date = 2024-11-06.
- Completion date =2026-07.
- 552 patients (females only).
- Start date = 2025-07-25.
- Completion date =2027-03.
Update: September 29, 2025
Early Study Finds Extended-Release Minoxidil Grows More Hair
An optimistic summary of the results from the 20-person Phase 2 trial of VDPHL01, with the volunteers taking 8.5 mg VDPHL01 twice daily for 4 months. In the control groups, 33 patients received 5 mg immediate-release oral minoxidil once daily for 6 months; and 34 patients received 1 mL of 5% topical minoxidil solution twice daily for 6 months. Investigator Global Assessment (IGA) was the primary outcome that was measured, and the IGA ratings were made by blinded investigators.
The summary contains an interesting quote from Dr. Jerry Shapiro (who it is mentioned has a financial relationship with Veradermics):
“A longer time to sulfate is what we want, so if we keep the drug in the blood longer, there is greater sulfation and more activity.
Make sure to read my past post on Minoxidil sulfotransferase boosters and why Minoxidil requires sulfation in order to grow your hair.
Also of note, VDPHL:
“Offers an opportunity to maintain drug levels above those needed for therapeutic effect, but below those associated with cardiac adverse events.”
Update: September 21, 2025
Veradermics Extended Release Minoxidil for Hair Loss: Superior to Topical and Oral Minoxidil
Veradermics presented its smaller 20-person Phase 2 trial results at this month’s EADV Congress in Paris. A post about this on Instagram is causing some excitement. It has two slides in there, and I will paste the main points below:
- The doctor Congress attendee who made the Instagram post states that “Extended release oral minoxidil has superior increase in hair density compared to topical minoxidil and oral minoxidil. Phase 3 trials are underway.” So this confirms that VDPHL is extended release oral Minoxidil, even though Veradermics’ pipeline page still does not declare the key ingredient.
- 82% of VDPHL01 patients achieved moderate to great improvement versus just 20% of topical Minoxidil or low-dose oral Minoxidil users.
- There is superior efficacy (3.5 times higher IGA scores) in a shorter time frame (4 months versus 6 months) compared to “competitors”).

Update: January 2, 2025
Veradermics Phase 2/3 Clinical Trial Enrollment Link
Veradermics Phase 2/3 US clinical trial enrollment link is live. Their 40 plus locations are pretty widely spread across the country. Please note that we are not certain of the active ingredient(s) in this tablet. From the company’s patent, I previously guessed that it is an extended release oral Minoxidil, plus hopefully some other surprises (see bottom of this post). But it could end up being something totally different too.
The study involves 13 visits to a clinic over the course of 12 months. Participants will either get the new treatment or be part of the placebo group (that will still also get a tablet).
Update: December 11, 2024
Veradermics Raises $75 Million for Phase 2/3 Trials
Veradermics just raised $75 million in Series B financing (h/t “meko”). They have also initiated a Phase 2/3 trial for their lead candidate VDPHL01 for the treatment of androgenetic alopecia (AGA). The trial will enroll 480 patients across 44 sites in the US. Note that Veradermics also has an ongoing 20-patient Phase 2 trial for VDPHL. The company plans to report topline data from that Phase 2 study in the first half of 2025.
August 9, 2024
Veradermics VDPHL Tablet: Phase 2 Trials Begin
Veradermics is a US-based startup that is working on a new tablet to treat androgenetic alopecia (AGA). They just started Phase 2 clinical trials for VDPHL01 in male subjects with AGA. Only 20 patients are enrolled, and the completion date is listed as August 1, 2025. The trial will take place at Therapeutic Research’s center in San Diego, California. Note that VDPHL likely stands for Veradermics Pattern Hair Loss.
The tablet does not impact hormone levels as do dihydrotestosterone (DHT) inhibitors finasteride and dutasteride. Thus avoiding potential side effects. Veradermics’ CEO is a young dermatologist named Reid Waldman.
Modified Release or Extended Release Oral Minoxidil
The mechanism of action (MOA) and key ingredient(s) in this tablet are both confidential. However, when I searched through the company’s patent, it seems like the drug candidate will be a “modified release” oral minoxidil tablet. In the patent, they use the term “extended release (ER)”. Note that they do not use the term “sublingual minoxidil” anywhere in the patent.
Other Potential Ingredients in VDPHL
In the patent, they also have a massive list of 191 claims. Within that section, all of the following drugs are listed 11 times each:
- Setipiprant (11 times).
- Valproic acid (11 times).
- Cetirizine (11 times).
- Medrogestone (11 times).
For long time readers of this blog, setipiprant (and Kythera) will ring a bell. It caused so much excitement a decade ago. I cannot believe that the very optimistic 9-yr old audio interview with Kythera’s CEO is still online. Setipiprant is an oral antagonist to the prostaglandin D2 (PGD2) receptor.
I covered valproic acid and hair growth in detail in the past. Follica also has a patent that covers valproic acid and hair regrowth. Valproic acid activates the Wnt/β-catenin signaling pathway.
Cetirizine is a PGD2 inhibitor that has been shown to benefit hair growth even when used topically.
I have never covered medrogestone on this site before. Per Wikipedia, it is a progestin that is an agonist of the progesterone receptor and a weak anti-androgen. Progesterone is a female sex hormone that has beneficial properties towards hair growth.
