At the end of March 2021, I read about a new South Korean company named Han Bio. Apparently, this company has managed to successfully develop a dermal papilla cell culturing technology.
Update: May 22, 2025
An interesting new interview with Han Bio’s chairman Da-witt Kang.
Update: April 9, 2025
Han Bio Aims for Phase 1 Clinical Trials in 2027
Han Bio is back after some prior delays and perhaps over-promises. In a press conference in Seoul on April 8, 2025, the company’s Chairman Kang Da-witt said that they would start Phase 1 clinical trials in 2027. Key quote:
“As the threshold for autologous cell therapy has been lowered with the revision of the Advanced Regenerative Biotechnology Act, we will collaborate with hospitals to promote rapid clinical entry and commercialization.”

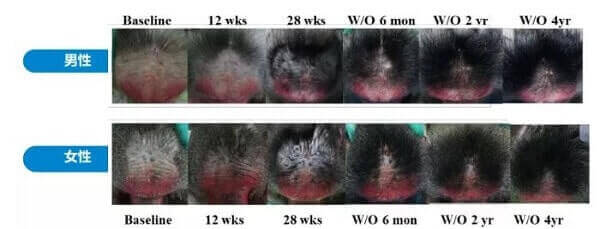
Of most significance, the company has developed a mammary papilla-based tissue engineering product called HSF-101, delivered via a new microneedle based system. In an experiment, it led to hair growth of up to 0.6 mm. Moreover, in mice, it led to the formation of new hair follicle structures.
Quote from Mr. Kang:
“We will become a company that conquers hair loss with mammary papilla cells.”

Interestingly, Mr. Kang mentions that his own hair loss bothered him and is what initially led to the creation of Han Bio.
Update: March 2022 — Hanmo Bio gets approval for manufacturing advanced biopharmaceuticals. Per CEO David Kang:
“We got approval of the cell treatment facility last year, the entry into preclinical trials, and the recent approval for the manufacturing of advanced biopharmaceuticals. We are now standing at a very important start line for the fundamental and complete solution of the hair loss problem that Hanmo Bio is planning.“
Update: October 2021 — Clinical trials will start in the first half of 2022. Han Bio will partner with Dt&CRO. The latter is South Korea’s first and only full-service contract research organization (CRO). Non-clinical trials will commence in November 2021.
Another article from March 2021, with CEO David Kang making a bold prediction:
“By collecting 50 to 100 healthy hair follicle tissues from the back of a patient suffering from hair loss, extracting the dermal papilla cells, cultivating them, and transplanting them back to the head, we can fundamentally cure hair loss.”
Of course at first I was incredulous. However, after further research, I decided that I should at least take the company semi-seriously. Han Bio’s blog is especially extensive. Some hair related articles on there go into many pages, so be sure to navigate thoroughly (after translation). Another version here seems to have additional news.
Note that when I contacted the company, they directed me to the above blog.
Han Bio’s Dermal Papilla Cell Storage and Culturing
Note that Han Bio is also called Hanmo Bio and Hanbio Group in news articles and on the company’s site. The externally located blog has some lengthy posts regarding this new technology. Some include images and video (screenshots further below).
In December 2020, Han Bio introduced its dermal papilla cell storage service called HDPC-480. The list price is 4.8 million South Korean Won ($4280 at current exchange rates). They offer a steep discount for the first 500 patients.
Note that in the western world, we have a similar option available via HairClone (UK). Moreover, HairClone has already been active for several years.
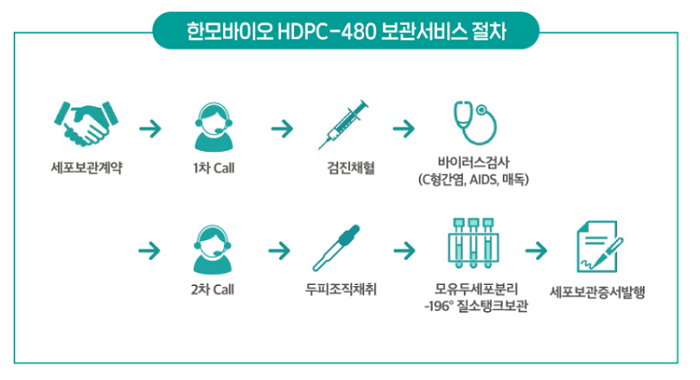
According to the balding CEO Kang Da-Witt (also called Kang David):
“Han Bio has secured technologies to separate and regroup the dermal papilla cells in hair follicles. It would take just a single strand of a patient’s hair to cultivate enough cells to reproduce around 30,000 hair strands.”
The company’s method is described as “chopping separation technology” after translation from Korean into English.
Han Bio has finished construction of its first plant, where the company stores and cultivates patient dermal papilla cells. These can then be cultured, multiplied and then implanted into the same patient via a hair transplant. This new plant will open by the end of May 2021.
Han Bio Clinical Trials and Patents

Han Bio will start clinical trials in the second half of 2021. According to CEO Kang Da-Witt, he expects that the time required for clinical trials will be shortened. This is due to a favorable new South Korean law which became effective on August 28, 2020. It is designed to help fast-track the market entry of regenerative medicine and biologics related products. Similar to Japan’s fast-track laws.
Han Bio is now preparing to apply for patents in the US, Japan and China. The are also open to out-licensing the technology.
Transplanting Someone Else’s Dermal Papilla Cells
From Han Bio’s extensive blog, one FAQ section caught my eye. It seems like the parent company of “Hanmo Bio” has successfully transplanted hair stem cells from one person to another. Without any immune reactions.

Obviously, this all sounds way too good to be true. But I have to prognosticate.
Asian countries dominate the world in the manufacturing of nano-scale semiconductors, microchips and integrated circuits. Perhaps our invisible-to-the-eye dermal papilla cells are next?
South Korea: Groundbreaking Hair Loss Treatments
South Korea is a world leader when it comes to plastic surgery and cosmetic procedures. When it comes to treating hair loss, South Korea is especially impressive in developing and/or using new technologies. For example:
- South Korea is one of only two countries in the world where Dutasteride has been approved for treating hair loss.
- The work of Dr. Kang-Yell Choi and CK Biotech in developing PTD-DBM to target CXXC5.
- PGD2 inhibition technology via Stemore (link no longer working).
- DKK-1 research and patent for locally-based Bioneer.
- My favorite: Allogeneic Person-to-Person Hair Transplants.
- South Korean companies are also at the forefront of developing stem cell related products, including AAPE and ADSC. Most such currently available products likely have limited to no benefits towards hair growth.
- Finasteride injections.
- Moogene.
- Epibiotech.
- OliX Pharmaceuticals and its RNAi based hair loss product.
- A disproportionate amount of research when it comes to hair growth from natural products. Especially from sea vegetables and algae.
Update: January 2022
South Korea’s President Says Government Should Pay for Hair Loss Treatments
South Korea is the first country in the world to suggest that the government should pay for hair loss treatments. Thank you President Lee Jae-myung. It seems like I am not as libertarian as I thought. Please see the middle of this post for much more on South Korea’s leadership in hair loss research.
Lee Jae-myung said on Wednesday that hair loss coverage is necessary from the aspect of “body completeness.”
 Read the bottom half of this post for my original report on Hope Medicine (China)’s HMI-115 (licensed from Germany’s Bayer). New updates are on top. This treatment targets
Read the bottom half of this post for my original report on Hope Medicine (China)’s HMI-115 (licensed from Germany’s Bayer). New updates are on top. This treatment targets