Update: May 24, 2024
Kangstem Biotech just announced that it has signed an agreement with HLB Biostep and HLB Biocode to establish an organoid-based drug safety and efficacy evaluation platform to develop cell therapy products. Among these will include a hair loss treatment based on skin organoids. Also of interest, a new study regarding skin organoid technology development that is co-authored by a Kangstem affiliated scientist.
December 28, 2023
Perhaps the best hair loss cure related news of 2023 just came out last week (h/t “Theo”). South Korea based Kangstem Biotech plans to test a hair cloning type of hair loss treatment in 2024.
The company will commercialize a cloned hair follicle-based drug screening and efficacy evaluation method; and begin nonclinical efficacy evaluation of hair transplants based on the cloned hairs.
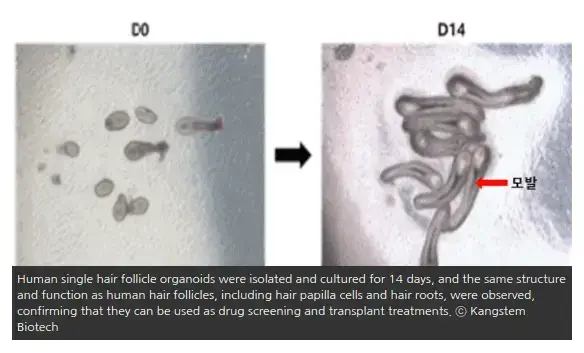
Kangstem Biotech to Commercialize Hair Cloning Treatment in 2024
Kangstem Biotech (South Korea) was founded in 2010 by Kyung-Sun Kang and is publicly traded. Its shareholders include a range of major Korean and Western companies. Without checking this company’s reputation, I might have delayed this post to next year.
The company specializes in cord-blood derived stem cell and other anti-aging related treatments. They are also a contract development and manufacturing organization (CDMO). I never heard about them till this week.
During the past two weeks, Kangstem Biotech had two press releases that are quite remarkable:
- On December 6th, the company announced plans to speed up the commercialization and launch of skin organoids to 2024. Interestingly, in 2021 they had an update about their artificial skin technology and partnership with Seoul National University.
- On December 22nd, the company announced plans to begin commercialization of the world’s first human hair follicle organoid-based hair loss treatment in 2024.
Per the second press release, Kangstem Biotech signed a contract with the Seoul National University Industrial Cooperation Foundation for:
“Human hair drug screening and human hair follicle production and culture technology for hair transplant materials to develop and commercialize hair loss treatment based on hair follicle organoids.”
Also check out the summary in Newsprime. And on Linkedin.
The company also states the following per the Korean to English translation:
“This technology is the world’s first artificial production of human hair follicle organoids in a test tube, and is a technology that reproduces human hair follicles.”
Kangstem has a two pronged approach when it comes to usage of its technology:
- Provide a drug screening platform for the development of hair loss or hair growth pharmaceutical treatments. They plan to launch their business for hair follicle-based efficacy evaluation methods in 2024.
- Begin non-clinical efficacy evaluation of using the hair follicles they culture for use in actual hair transplants. Also in 2024.
The government regulations for regenerative medicine in rapidly aging developed Asian countries have become very flexible. South Korea cannot afford to wait too long, considering that average birth rates in the country hit just 0.72 children per woman in 2023.
Also of significance, South Korea and Japan are both trying to become world leaders in cosmetic procedure related tourism.
South Korea’s Leadership in New Hair Loss Treatments
This adds yet another new South Korean entrant in the hair cloning or hair multiplication sector. Others that I have covered recently include Epibiotech and Han Bio. For a list of all major South Korean entities that are working on any kind of important hair loss treatment, check out my page on hair loss cure research around the world.
Update: Below is an e-mail update to me on 12/29/23 from “Theo” —
“From the press release I understand that they will commercialize hair follicle/skin organoids for drug testing by the first half of 2024. In parallel they will test the hair cloning technology in non-clinical trials to confirm efficiency (if I understand correctly). This technology is similar to Stemson, because both companies works with iPSCs and in vitro, and Stemson is at least 10 years away from commercialization.
Non-clinical testing in South Korea is the first step required by the state, and then comes clinical trials.
Guidelines for clinical trial approval (CTA) for drugs:
https://credevo.com/articles/2017/09/25/south-korea-clinical-trials-regulatory-process/
This is a very well established company, very well funded, with high quality infrastructure and connections. So it should go very fast.
The main CEOs and scientists in KangStem come from Seoul National University, which is in the top 25 universities worldwide.”
Well, by the time I get a consultation with the God that is Dr. Zarev, this may be close to realization, lol.
Nice work Admin,! But the only concern for me personally is how safe is such a treatment? We don’t know if severe side effects can manifest years down the line. Unless I’m missing something of course.
Great end to year! Maybe they will only test in Koreans next year, but still good stuff.
One more news item from 2023:
https://www.google.com/amp/s/www.koreabiomed.com/news/articleViewAmp.html%3fidxno=20462
So fast! This is exciting, whether successful or not. Because it could boost global hair loss research
I’m sorry if this is a stupid question: but what exactly is being commercialized? Is it a cloned hair follicle solely for drug screening and evaluation or an actual functional hair follicle? As exciting as this is potentially, I’d like to temper my expectations.
I second this question.
Are they commercializing a service that has ready to implant hairs this year? Meaning unlimited donor hair for transplants?
Or are they commercializing “human hair drug screening”, whatever that means?
Max, noone truly knows. It seems to be a sham.
Can you update in what Phase Han Bio is actually? I thought they planned to launch a Cosmeceutical in 2023 ??
HanBio is quiet since March 2022. It was a scam all along, the claims and timelines were ridiculous – but I think most gave them the benefit of the doubt.
Kangstem I have no clue whatsoever. I became very wary about companies who „suddenly“ offer treatments that will be available soon.
Drug screening in organoids/follicles is already being pursued by Fukuda and Epibiotech, Monasterium has similar methods. But I have no idea if these models are already being applied in reality or if they are in development only, and what the exact differences of these models are.
Epibiotech: http://epibiotech.com/en/service/
Fukuda: https://www.researchgate.net/publication/369505695_In_vitro_hair_follicle_growth_model_for_drug_testing
Paus: https://www.monasteriumlab.com/services/pre-clinical-research/models/human-hair-follicle-organ/
So unfortunately I don’t see the big news in Kangstem here. Furtheron: I highly doubt them to provide hair follicles for transplantation any time soon. This endeavor is just huuuge, and took Stemson, Fukuda, Epibiotech, Tsuji years and years of R&D – yet unsolved.
And my last point: there were quite some sketchy claims and dubious companies coming out of SK (not only hair-related) in the past years. It’s a different culture, and it’s very important to be successful, and if you’re not, you appear to be successful. This applies to academia too. Press releases, cooperations, announcements, collaborations, research papers,…the noise is important.
I‘m not saying Kangstem is fraudulent at all, as they are publicly listed and were founded many years ago with quite a number of employees too. Let’s see what they are cooking in 2024.
Anyhow, nice find – every new player is welcomed.
Agreed.
Their size and long-time partnerships with the major local university makes it seem like there is some potential that they are not entirely a scam.
The guy “Theo” who often e-mails me about Asian companies said the following:
“Main CEOs and scientists in KangStem come from Seoul National University, which is in the top 25 universities worldwide.”
On an unrelated note, a new study from South Korea that was posted today on Twitter:
https://www.nature.com/articles/s41598-023-49510-6
FYI — I posted a new update from “Theo” at the bottom of the post. Interesting link in there that is worth exploring.
Also embedded a video presentation link that Theo sent (it starts in the relevant time stamp).
Hello, admin.
Is Kangstem working on a “CURE” for baldness? I mean are they talking about beginning the trials of hair cloning?
Please clarify. Also please share updates about EpiBiotech, whether they are planning to launch a baldness “CURE” (or hair cloning)? Please share the latest updates.
Thank you.
I’m speaking in general
most scientists talk about various theories for hair growth
but none of them made a cure that works and that we have
For example, find the theory of making an atomic bomb on the Internet
Do we know how to make it?
Of course not
That’s how they say and say that they’d rather be involved in politics than scientific work
So if I’m understanding correctly, first trial in 2024 is not clinical (as required). If all goes well, there will then be a clinical trial. Makes sense and I’m sure they’ll fast track it (again, if all goes well – we can’t assume anything). Fingers crossed the clinical trial isn’t five years out.
The goal is to provide cloned hair (your own cloned hair) for hair transplants, right? If so, it’s exactly what I’ve always said a cure would look like. Forget donor hair – most people don’t have enough to get them to where they want to be (once you do a HT with 3-4k grafts you quickly realize (or realize a few years later) how many more you truly need, depending on your situation – it’s really not a ton of hair in 3-4k grafts, it just sounds like a lot). And those can thin as well over time (regardless of what they say).
I hope it works well, I hope it’s fast tracked and I hope the cost is reasonable. I’ll take 10-12k grafts right now. My biggest issue is my hairline (it has retreated to the point my forehead is a football field).
The stock had a good December, but it’s still down 85% in last 5 years, which shows there is no institution confidence in this company.
It’s right inline with Kintors 5 yr performance of -88%. Unfortunately, both of these companies are likely frauds.
New updates:
https://www.linkedin.com/posts/kangstem_%EA%B0%95%EC%8A%A4%ED%85%9C%EB%B0%94%EC%9D%B4%EC%98%A4%ED%85%8D-%EC%9D%BC%EB%B3%B8-%EC%A4%84%EA%B8%B0%EC%84%B8%ED%8F%AC-%EC%9E%AC%EC%83%9D%EC%9D%98%EB%A3%8C-%EC%82%AC%EC%97%85-%EB%B3%B8%EA%B2%A9%ED%99%94-activity-7156067708810272769-w-OG
Thanky for your replies. I never check LinkedIn, not my cup of tea…but it seems like some companies use it as a primary means of communication.
Anyhow, here’s another article regarding Kangstem, not sure, could be the same as on LinkedIn:
https://www.medipana.com/article/view.php?news_idx=322049&sch_cate=D
Maybe I underestimated Kangstem, they are doing a lot of R&D (look at the cartilage regeneration, extremely impressive!).
Yes same article. That Linkedin page I posted should open even if you are not logged into Linkedin.
New Kangstem update on the company beginning Phase 1 osteoarthritis related clinical trials.
Some interesting news in there about the pending amendment to South Korea’s Advanced Regenerative Medicine and Advanced Biopharmaceuticals Act. Will really speed up time frames.
https://kangstem.com/pr/ir_news/view?i=625
I have become excited about this company again, simply by seeing the photo (if it is real) of the hair follicle organoid… although their plans to carry out clinical trials are not soon, at least if they are ever carried out they will be exactly what they say: implant artificially created hair follicles into bald scalps
This is the cure if you ask me. Other things may help but even transplanted hair can fall out. Implanting artificial follicles into scalps is the way to go. I’m surprised it’s taking this long though, honestly.
In South Korea, world’s lowest fertility rate plunges again in 2023:
https://www.reddit.com/r/Futurology/comments/1b22suf/in_south_korea_worlds_lowest_fertility_rate/