For the second time in just two months, the global media has gone beserk over photos that show complete reversal of alopecia areata. This time via ruxolitinib. The first time was two months ago in one of Dr. Brett King’s patients via the rheumatoid arthritis drug tofacitinib.
Ruxolitinib and Alopecia Areata
This time, it is via the bone marrow cancer drug ruxolitinib (approved for sale in the US and EU under brand names Jakafi and Jakavi). It seems like ruxolitinib can cure hair loss in some people suffering from alopecia areata.
These current results are even more significant because:
- They occurred in three patients rather than just one.
- The research and findings were conducted by the renowned hair loss research expert Dr. Angela Christiano and her team (in particular, Dr. Raphael Clynes) at Columbia University. In 2013, Dr. Christiano had already presented findings that both tofactinib and ruxolitinib reversed alopecia areata in mice. Moreover, these two doctors already filed a patent related to JAK 3 inhibitors in 2012 and are bonafide experts in this field.
- These new findings also determine the cellular mechanism (certain set of T cells attack hair follicles) that causes hair loss in people with alopecia areata. This was something that was not completely understood in the past.
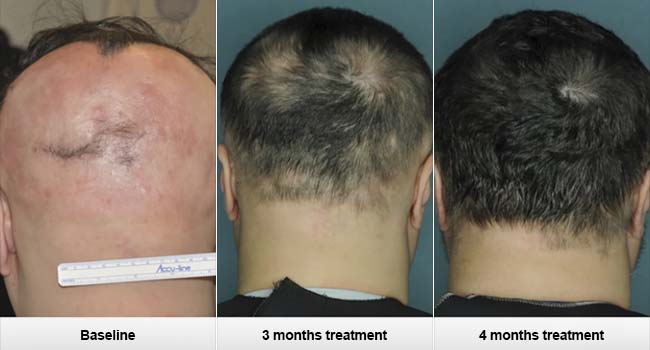
The current findings were released in the online edition of Nature Medicine yesterday. All three patients saw excellent results within 4-5 months after taking the twice a day pill, and no adverse side effects were reported.
One of the patient’s images released by Columbia University:
Video from Columbia University:
JAK Inhibitors
Just as I clarified two months ago when the tofactinib results came out, these results are yet gain unproven to work on the majority of balding people. This is because over 95 percent of hair loss is caused by a hormone driven condition called androgenic alopecia, rather than this less common alopecia areata problem.
However, no decisive study has yet been undertaken that examines the impact of JAK inhibitors such as tofactinib or ruxolitinb on people with androgenic alopecia. So the case is far from closed. I think that there is a good chance of there being an inflammatory immune system attack component to androgenic alopecia for many people, especially those who have significant itching and dandruff associated with their hair loss.
These drugs are expensive, and potential side effects are far more serious than with a drug such as Propecia. So doctors are reluctant to conduct these studies for what they feel is a cosmetic problem. However it is only a matter of time (probably months rather than years) before small-scale studies will be undertaken on all balding people.
Partly due to this development, Mark Blake of The Trichological Society of the UK said the following yesterday:
“It is a matter of time before we find a cure for male pattern baldness as well as alopecia. We know so much more about hair today and how it grows. We would like to think a cure could come within five years, definitely 10.”