There are several interesting new updates in regards to Minoxidil Sulfotransferase Booster news. Thanks to the reader comments.
September 24, 2023
Minoxiboost Minoxidil Booster Released
Minoxiboost solution (60 ml) finally got released in India via Cosmofix. It is currently only sold in India via Cutiscart. The official July 25, 2023 launch announcement from Jupiter Wellness (US) mentioned its Indian partners Sanpellegrino Cosmetics and Cosmofix Technovation. The two of them have exclusive distribution rights to this product in 31 countries in Asia and the Middle East.
Also of interest, a company named Rida Hair Research Institute (US) is selling its own Minoxidil booster spray product on Amazon (Edit: Now removed). The call it Mino Boost and it currently has an average rating of 4 out of 5 stars based on 62 reviews. They claim that it will make your topical Minoxidil treatment 7X more effective. The sale of this product began in February 2023 per Rida’s Instagram.
A more expensive Minoxidil booster product is being sold in the US by Daniel Alain.
Note that compounded Minoxidil Sulfate (MXS) based topical formulations are also available for medical use in some parts of the world, such as Brazil.
January 19, 2023
The Minoxidil Sulfotransferase Enzyme
With oral Minoxidil becoming the most discussed hair loss treatment over the past year, now is a good time to discuss the Minoxidil Sulfotransferase (SULT1A1) enzyme.
The below diagram is from a Polish study published in 2022 covering Sulfotransferase activity in hair follicles and the response to Minoxidil treatment. Sulfotransferase 1A1 is encoded by the SULT1A1 gene.
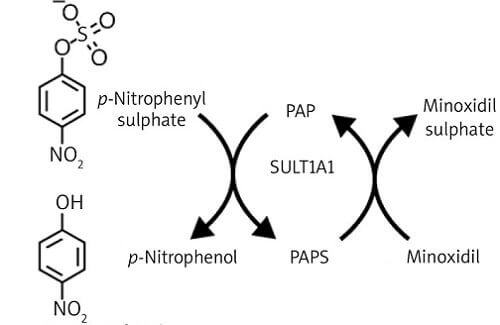
When Minoxidil is used topically on the scalp, it is converted into its active form (Minoxidil sulfate) by SULT1A1 enzymes, which are located in the follicular outer root sheath. However, many people’s hair follicles lack high levels of sulfotransferase. So these men (and women) are unable to convert even extra strength Minoxidil into its active form that stimulates hair growth.
Note that Retin-A (Tretinoin) enhances the response to Minoxidil in androgenetic alopecia patients by upregulating follicular sulfotransferase enzymes. No wonder so many blog readers have recommended the use of both products together.
Benefits for Oral Minoxidil too?
When it comes to oral Minoxidil, the body’s liver does the job of converting Minoxidil into Minoxidil sulfate (also spelled sulphate). Several articles that I read seem to suggest that the hair follicle does not need to do the conversion anymore. However, Dr. John Cole said the following to me:
“If you can’t sulfate it, Minoxidil will not work. Peripherally sulfated Minoxidil cannot enter the cell.”
Also, in this 2020 letter from Ramos et al (full version here), a key quote:
“Increasing the activity of SULT1A1 can improve the efficacy of topical and low dose oral minoxidil for the treatment of AGA.”
Moreover, the following quote in last year’s important article from the AAD in regards to LDOM suggests some uncertainty via the use of the word “may”:
“It has been suggested that oral minoxidil may also be converted by liver and platelet sulfotransferase, thereby reaching a higher follicular accumulation.”
It would be interesting to know if beard growth with topical Minoxidil is also impacted by SULT1A1.
Boosting SULT1A1
In recent years, there have been some significant efforts made to develop products to boost the Sulfotransferase enzyme in order to improve the efficacy of Minoxidil. Leading the way is Dr. Andy Goren, who started a company called Applied Biology that created a Minoxidil Booster product.
However, in June 2021, Applied Biology was acquired by Jupiter Wellness. Luckily, while researching for this post, I came across Jupiter’s hair loss product page. Lo and behold, some interesting news on their Minoxidil Booster topical product:
- It has been clinically shown to increase the sulfotransferase enzymes needed for minoxidil to work by up to 7x over a two-week period. I assume this 2021 study pertains to those findings. Co-authors include the well known hair researchers Dr. Rodney Sinclair and Dr. Rachita Dhurat.
- The product has already been licensed to Taisho for the Japanese market. Taisho is Japan’s leading seller of Minoxidil products. They expect to launch the product commercially in 2023.
- It is also licensed to Cosmofix Technovation and Sanpellegrino Cosmetics, both from India.
Note that in the study that I listed in the first bullet point above, 75% of patients in the SULT1A1 group demonstrated a positive increase in hair growth; compared with only 33% of patients in the placebo group. However, the study limitations include its small sample and limited duration of follow up.