I originally added this story about Ideeea Therapeutics (Spain) as an update to my past post on mesenchymal stem cells (MSCs) and hair growth. However, lots of readers missed it, and this company keeps getting widespread global media coverage. So I have now written this separate post on Ideeea Therapeutics. If you made a comment in that post, feel free to re-post it here, and I will then delete it from there.
Ideeea Therapeutics AGAcell: MSCs and ATP
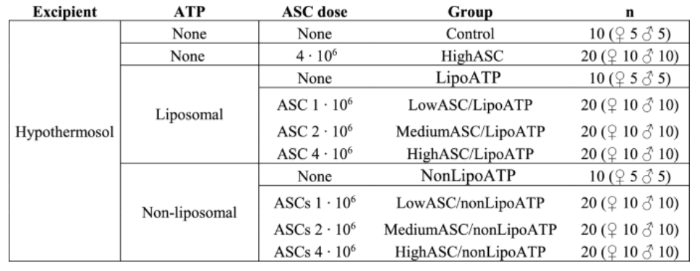
Spain-based start-up Ideeea Therapeutics has raised €2.3 million in seed round funding. The company’s AGAcell ® technology entails the treatment of androgenetic alopecia via the intradermal administration of a patented formulation. This consists of allogeneic mesenchymal stem cells (derived from adipose tissue) combined with a “bioactive molecule”.
The bioactive molecule entails adenosine triphosphate (ATP). The current research was conducted successfully in mice that were treated with various iterations of MSCs and ATP after being induced to lose their hair via the administration of dihydrotestosterone (DHT). Both liposomal and non-liposomal ATP were tested, along with various doses of adipose-derived stem cells (ASCs). The various doses gave different outcomes in male versus female mice, but ultimately, the results were almost always positive:
“The researchers explained that the injection of low-dose stem cells and ATP into male mice resulted in a nearly 100% hair growth effect in three weeks. In female mice, there was no effect at high or low doses, but the hair growth rate reached 90% at medium doses.”
The combination of adipose derived stem cells and adenosine triphosphate regenerates hair via the regenerative capacity provided by the former, and the cellular energy increase provided by the latter. In this study, the authors conclude that:
“ATP supplementation of cell preparations might be a good therapeutic option to enhance the beneficial impacts of MSCs.”
Dr. Eduardo López Bran, the lead scientist for this treatment, was interviewed on June 1 and also covered in this article on June 5. He is the head of the Dermatology Department at the San Carlos Clinical Hospital. He expects that human clinical trials could begin in 2027 or 2028, with an estimated duration of two years. However, the company’s pipeline page indicates that Phase 1 would begin in 2025/2026 and Phase 3 would end around 2030.
Note that in 2024, I covered a Taiwanese company that is working on an AMPK activator product to boost ATP levels of human follicle dermal papilla cells. ATP sprays are often used during hair transplant procedures. And laser hair growth device energy also increases production of ATP.
Also of note, one of the main ways in which Minoxidil works to grow hair is via the opening of ATP-sensitive potassium (KATP) channels.



