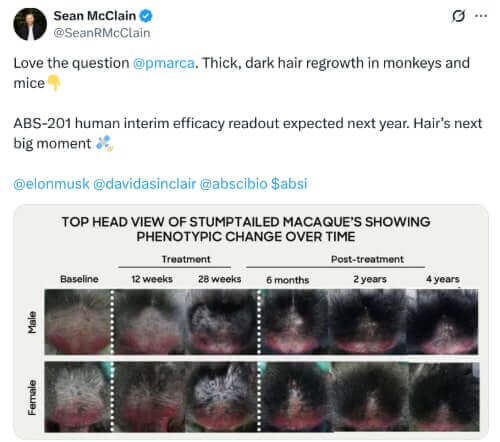
October 23, 2025
Finally, we have an interesting update on allogeneic hair transplants, with a panel discussion at this week’s ISHRS 2025 conference. The below introductory video from the ISHRS is worth a watch.
January 13, 2019
One of the most common questions that people suffering from hair loss ask is why one cannot perform a hair transplant from one person to another? i.e., an allogeneic hair transplant, which I first discussed in 2015.
If this were possible, it would essentially be a hair loss cure. There would be no shortage of people with stellar hairlines that would be willing to donate a fraction or their hair to balding men and women for a decent price. My father would give me some of his for free.
Unfortunately, the biggest issue with person-to-person hair transplants is rejection of foreign material. The only way to overcome this problem would be via the recipient taking immunosuppressants for life, which is potentially very dangerous.
Allogeneic Hair Transplant Success: No Immunosuppressants
A few days ago, it was announced that researchers from Seoul National University Hospital (SNUH) had successfully conducted an allogeneic hair transplant without the use of immunosuppressants. The research team was led by Professor Kwon Oh-sang.

While this particular success involved 24 mice-to-mice hair transplants rather than person-to-person hair transplants, it is still absolutely groundbreaking.
Moreover, the mice immune systems were “humanized” via hematopoietic stem cell transplantation.
Nevertheless, this story was not covered by any newspapers or online publications in the western world. No idea why as some of them (especially the superficial Daily Mail) have excellent coverage of groundbreaking hair related stories.
Normal hair transplants as we know them are autologous. Meaning that a person has his own grafts moved from his donor site to his recipient site. Allogeneic hair transplants are an entirely different animal.
Remove the Dendritic Cells
In this latest South Korean research, the scientists overcame immune system rejection of donor hair by eliminating dendritic cells. The team used ultraviolet B radiation to remove all the donor dendritic cells that were present in the donor hair follicles.
Interestingly, the scientists point out that hair follicles are less likely to be rejected by the immune system than other organs such as the heart, kidney and so on. In this regard, hair follicles are a bit like the cornea in terms of immune privilege.
According to the team, hair follicles are independent organs present in the skin and have an “immune privilege” that is relatively free from immune rejection. As the brain and cornea also have this privilege, the team could reproduce the same state of hair follicles that existed in people’s bodies by removing the donor dendritic cells involved in direct antigen presentation.
According the Dr. Oh-sang, such an allogeneic hair transplant procedure will be challenging to apply in practice when it comes to humans. However, this discovery does create new potential applications that were not possible before.
I wonder if he plans to start experimenting with part-autologous and part-allogeneic hair transplant procedures in humans in the near future? Most likely, donors will be selected via Human Leukocyte Antigens (HLA) matching.
Also of Interest:
— First ever skull and scalp transplant.
— Face, organ, and limb transplants: and immunosuppressive drugs.