I have covered hair regeneration research by Yokohama University’s Dr. Junji Fukuda a few times in the past. He is highly respected in the world of hair loss research.
Preparation of Hair Beads and Hair Follicle Germs
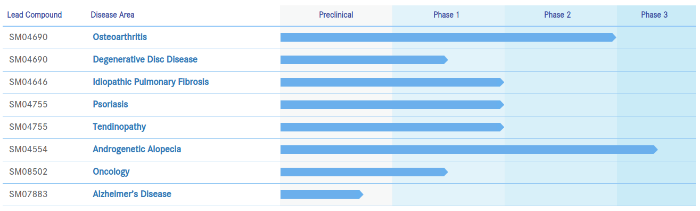
Last month, Dr. Junji Fukuda and his team published a groundbreaking new study titled: “Preparation of hair beads and hair follicle germs for regenerative medicine“. The team managed to generate new hair follicles from stem cells in far higher quantities than ever before in mice. This news was widely covered, including in today’s Guardian newspaper.
The researchers’ approach entailed use of a collagen gel in combination with spheroids formed from epithelial cells. This resulted in something called bead-based hair follicle germs (bbHFGs). These germs generated hairs more efficiently than previous approaches. A hair-raising protocol per one headline.
The success of this approach will potentially lead to the preparation of microtissues with high trichogenic ability upon transplantation. A key necessity in future hair follicle germ creation and transplantation from stem cells in humans.
Past research from Dr. Fukuda is here. The researchers next step is to “find a way to expand the number of hair follicle stem cells“.
The below post on earlier work from Dr. Fukuda was written in February 2018.
Large-Scale Production of Hair Follicle Germs
I have covered Dr. Junji Fukuda and the Fukuda Lab several times on this blog in the past. Their important hair related research takes place at Yokohama National University in Japan. Most recently, this was just two weeks ago in relation to their latest paper titled: “Spontaneous hair follicle germ (HFG) formation in vitro, enabling the large-scale production of HFGs for regenerative medicine“. It was published towards the end of 2017, but seems to now be dated as 2018.
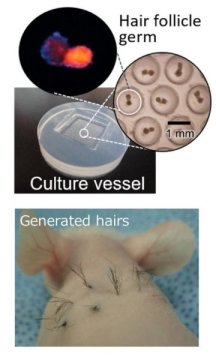
At the time, I decided that this development was only worth a cursory mention in my once a month “brief items of interest” post. My decision was clouded by the fact that this research only entailed work on mice (see bottom part of image on left, courtesy Yokohama National University). We are all a bit bored/tired/frustrated with that of course.
However, earlier today, Dr. Fukuda and his team’s work was covered in the Science Daily publication. Key quote from Dr. Fukuda:
“This simple method is very robust and promising. We hope that this technique will improve human hair regenerative therapy to treat hair loss such as androgenic alopecia,” adds Fukuda. “In fact, we have preliminary data that suggests human HFG formation using human keratinocytes and dermal papilla cells.”
Perhaps this really might end up being a major development, even in humans.
Tsuji, Shiseido, Ohyama and perhaps now Fukuda? I have never been to Japan, but it looks like this may change in the future.