When I published my first post on exosomes and hair growth in late 2019, I got numerous requests for an update. However, the US FDA has since posted warnings about the unregulated use of exosomes for hair loss and other regenerative medicine applications. I was awaiting further updates in relation to product safety and legality.
Over the past decade, the FDA has generally been very hands-off when it comes to stem cell and related treatments. This includes PRP, adipose-derived stem cells, SVF and more. The government likely did not want to inhibit the growth of new therapies in regenerative medicine per Dr. Ron Shapiro.
However, we now have problems with the proliferation of unethical clinics. Some use third-party derived exosome products that are poorly processed and can cause side effects and infection. Sometimes, these products may not even contain exosomes! Or they contain 1000s of times fewer exosomes per cubic centimeter (cc) then promised.
One key issue to look out for in 2021 will be updates on 351 versus 361 status. The former is expensive to attain. Technically, exosomes fall under 351. Meaning that they need to be regulated as drugs and/or biologics. Note: I am not 100 percent certain about these technicalities, but the gist of the matter is that one needs to be cautious.
Adding to my delay in writing this post, the initial excitement about this subject matter was perhaps overblown. Exceptional results have not been as frequent as expected and advertised in some Youtube videos out there. I also wanted to see if more doctors would jump on the exosome bandwagon in 2020. And if more studies and patents would be published on this topic.
Exosomes for Hair Loss Feedback
I have received feedback and updates from a number of doctors since I published the original post in 2019.
Dr. Jerry Cooley is one of the early pioneers of using exosomes to treat his hair loss patients. His website has an updated section on this subject with a number of before and after photos. Dr. Cooley uses ExoFlo™ products from Direct Biologics. These exosomes are derived from bone marrow cells. Note that Direct Biologics also market the product as XoFlo™.
According to Dr. Robin Unger, her exosome results are more dependable than platelet-rich plasma (PRP). Moreover, she already gets good results from using PRP to treat hair loss. Make sure to compare growth factors in PRP versus this comprehensive list of growth factors in exosomes. Dr. Unger has been using exosomes for more than a year and is currently undertaking a study on their benefits.
According to Dr. Jeffrey Rapaport, “Exosomes are not FDA approved. No guarantees or promises can be made regarding treatment or cures of conditions or diseases with exosomes”. He only uses them for people who have not responded as well as he likes to PRP. Below is one of Dr. Rapaport’s patients before and 3 months after being treated with exosomes for his hair loss.
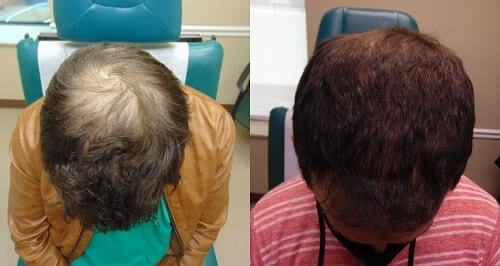
Dr. Rapaport also said that he believes in Direct Biologics. “The company has an IND for using exosomes to treat Covid. This says something for the safety of the product”. Per the doctor, one cannot be sure that exosomes will still be available to treat hair loss after May of 2021. Apparently, that is when the FDA concludes its evaluation.
Note: My hope is that the FDA takes a lenient position on exosome use. But also imposes very stringent requirements on any company seeking proprietary exosome product marketing approval. If there is an outright ban, I would not be surprised if people start going to other countries for treatment. This would be far more dangerous.
According to Dr. Ron Shapiro, the debate over the use of placental versus bone marrow mesenchymal stem cell-derived exosomes still exists. Note that stromal and mesenchymal stem cells (MSCs) can be derived from bone marrow; the placenta; umbilical cord tissue; adipose tissue; molar cells; and amniotic fluid.
Combination Treatment with PRP
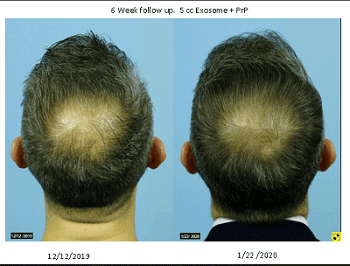
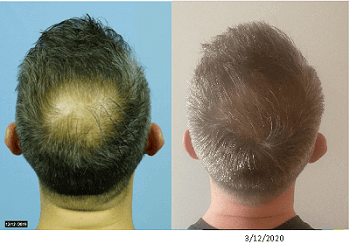
Dr. Shapiro is very pleased with his results to date and sent me the below two before and after photos of one of his patients. Note: I always assume that all photos sent to me from any doctor or surgeon are examples of best-case results.
The first photo below is 6 weeks after combination treatment with 5 cc exosomes and PRP. The second photo is another 6 weeks after an additional 5 cc exosomes + PRP + Wharton’s Jelly addition to the treatment.
The results are excellent in my opinion. Note that the second after photo was taken by the patient himself at home, hence the difference in lighting. It is often not possible to get a patient to fly back to a clinic just to get an after photo in similar lighting and background. Dr. Shapiro also sent me closeups of the crown and entire frontal scalp of this patient. All looked great to me.
Week 0, Week 6 and Week 12 Treatment Progression
New Exosome and Hair Loss Studies
In comparison to 2019, fewer studies on exosome treatment for hair loss were published in 2020. But at least half of this year was wasted due to shutdowns.
- An August 2020 summary from China on stem cell-derived exosomes and conditioned medium (CM) for hair growth.
- A July 2020 report from the US on how dermal exosomes promote hair regeneration by regulating β-catenin signaling.
- A 2020 literature review with several before and after photos of successful results.
And finally, a September 2020 article on stem cell hair loss treatments has two important notes on exosomes:
- There are currently no clinical studies on extracellular vesicle (or exosome) therapy for hair growth.
- There are presently no standard effective isolation methods for exosomes.
The second statement above ties in with recent warnings from the US FDA. Irreputable exosome products can cause major side effects or worse.
Part 2 of this post will come next week.