Nanfang Hospital, Southern Medical University
In 2015, I discussed new hair multiplication related collaborative work between the University of Manitoba (Canada — led by Dr. Malcolm Xing) and Nanfang Hospital Southern Medical University (China). Researchers from the latter recently published two new hair related studies, one of which analyzes the results of Chinese clinical trials involving mesenchymal stem cell injections for hair growth.
Note that hair follicle derived mesenchymal stem cells (HF-MSCs) include both dermal papilla (DP) cells and dermal sheath (DS) cup cells. Mesenchymal stem cells can also be derived from: adult tissues (bone marrow, peripheral blood, adipose/fat and teeth); and neonatal-birth associated tissues (Wharton’s jelly, placenta, cord blood, umbilical cord and amniotic fluid).
In recent years, mesenchymal stem cells have been in the news a lot due to the rising popularity of mesenchymal stem cell (MSC)-derived exosomes in regenerative medicine applications.
Treating Androgenetic Alopecia with Mesenchymal Stem Cell Injections
Recently, reader “Theo” sent me a link to a new November 2024 study from the Department of Plastic and Aesthetic Surgery, Nanfang Hospital, Southern Medical University. It entails a superior method of isolating human hair follicle dermal papilla cells (DPCs).
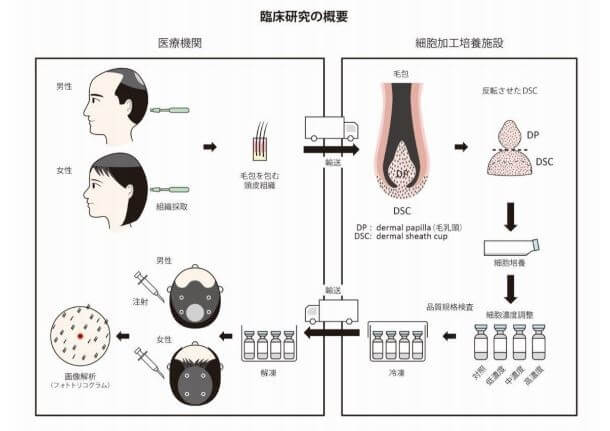
More importantly, several of the co-authors of this study also co-authored a September 2024 study titled: “A Clinical Trial of Treating Androgenic Alopecia with Mesenchymal Stem Cell Suspension Derived from Autologous Hair Follicle.” This trial occurred at the Department of Plastic and Aesthetic Surgery, Nanfang Hospital, Southern Medical University (China). The link to the actual 50-person trial that ended in 2022 can be seen here.

The results were encouraging, although the increasing hair count and hair thickening effects lasted for just 3 months. Note that at around 7 months, the treated group still had slightly higher terminal hair counts and mean hair diameters compared to when they started out. If this method is improved, a once-a-year injection session is totally fine by me.
“An increased proportion of terminal hair and hair shaft diameter was observed in the experimental group at 1 month. The effect lasted for 3 months. The hair-thickening effect of advanced miniaturized hair follicles with hair shaft diameter less than 60 µm was more notable than that for above 60 µm. No patient experienced any obvious side effects.”


They also include two images showing before and after (3 months) hair growth results. The left side in the below image is the before photos.

Stem Cells and Hair Multiplication
This makes me even more positive about Shiseido’s dermal sheath cup cell hair multiplication procedure that was released in 2024. And perhaps HairClone will finally test its dermal papilla cell injection treatment on humans in the UK in 2025. Also of note, in 2024, Dr. Junji Fukuda of Fukuda Lab announced that “Dermal papilla cell transplantation is about to begin in Japan.“
And several South Korean hair loss companies could test this in humans in 2025 too. In the past, Aderans (Japan) did the same with some success.
Note that similar attempts have been done in animals and humans in the past. Further reading:
- An April 2023 literature review of stem cell applications in human hair growth.
- A March 2023 systemic review on human stem cell use in androgenetic alopecia.
In my 2015 interview with Dr. Xing, he said that he had just returned from a trip to Nanfang Hospital and that:
“A team of more than 10 clinical doctors is working on hair loss in the Department of Plastic Surgery, Nanfang Hospital.”
It is always great to see more hair related research and clinical trials taking place in China. Within East Asia, the country has lagged Japan and South Korea in importance when it comes to hair research.