Update: July 2, 2018 — Histogen has just kicked off Phase 1 trials for its female hair loss product HSC660. The below post was originally published in May 2018.
When it comes to the speed of progress in a company developing and bringing forth to market a hair loss product, the most disappointing company for me has been Histogen.
Replicel and Follica have also been major letdowns in their speed of progress, although the former is perhaps now reliant on its partner Shiseido for further progress. Follica has made some big statements via its new and improved website during the past year, fingers crossed. In earlier years, the biggest disappointments were Aderans and Intercytex.
Histogen Background
I first covered Histogen in 2013, just a few months after starting this blog. In that post, I said that Histogen’s “male hair loss product (Hair Stimulating Complex — HSC) is in phase 2 trials, while the female product is yet to enter phase 1 trials“. Around the same time, I happened to be in San Diego and even visited Histogen’s offices and very briefly talked to a key staff member. I am clearly into hair loss research (anywhere in the world).
Note that Histogen’s product supposedly increases hair count via the injection of key growth factors in the form of KGF, VEGF, follistatin, placental growth factor, angiogenin and hepatocyte growth factor.
Since that original post, I have covered Histogen numerous times on this blog, with most developments entailing either 1) Conference presentations from CEO Gail Naughton; or 2) Brief mentions of Histogen getting some new financing and funding. In one of my past posts about Histogen, someone from the company even replied to a reader comment on this blog.
The one exception to the above was this upbeat post from 2016, in which we learnt from Dr. Naughton that Histogen’s HSC treatment would be made available in Mexico first (in 2018). They were also planning to soon conduct a large-scale 330-person clinical trial in Mexico with a local partner. They were also close to getting a partner in China for further clinical trials.
In 2017, Dr. Naughton then said the following in an interview:
“The U.S. trials are planned to commence in 2018; we expect it to gain approval in Mexico first, perhaps in 2020, and then in the U.S. sometime after that.”
And now in April 2018, Dr. Naughton said:
“Naughton said Histogen also is moving toward a late-stage clinical trial in Mexico for use of its HCS in men for treating baldness.”
US FDA Approval for Histogen IND for Females
Earlier today, I got a bunch of alerts in my e-mail about Histogen. The big news is that the US FDA has just approved Histogen’s Investigational New Drug (IND) for Female Hair Loss Trial. For those who do not know, an IND is the first step before Phase 1 clinical trials, and primarily focuses on drug safety.
So 11 years after Histogen was founded, they are possibly soon entering Phase 1 clinical trials for their female hair loss product. Makes one want to cry, but perhaps they will be able to speed these trials up due to already having tested the product in males. In their latest press release, they call their Hair Stimulating Complex product “HSC660”.
The only reason I decided to write this post was because of the below encouraging paragraph from this latest press release:
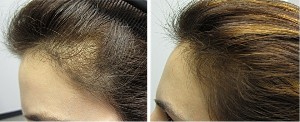
“Pilot and Phase 1/2 Clinical Trials of an HSC660 predecessor were completed in male pattern baldness outside the US, with results that produced statistically significant efficacy indicators and a clear safety profile. More recently, a physician-sponsored 10 patient study in the US showed cosmetically significant results in both men and women. In addition to seeing a 100% female responder rate in the physician-sponsored study, previous trials have shown efficacy in other difficult-to-treat populations including men over 40 years of age and temporal recession hair loss”.
