
I have covered topical finasteride at least ten times on this blog. My original post on this subject got published in 2014, and is updated every year.
I have especially emphasized Polichem’s P-3074 (now Almirall’s ALM12845) hydroxypropyl chitosan (HPCH)-based topical finasteride. This product is now finally being released in the European Union countries. Note that besides chitosan, there are many other potential delivery systems for topical finasteride.
Unlike specialized topical finasteride products offered by local compounding pharmacies or hair clinics, Almirall’s ALM12845 has undergone rigorous three-phase clinical trials. For more information, see Phase 3 trial results of P-3074 as well as this 2015 encouraging update.
Update: March 21, 2023 — Boryung will launch Finjuve finasteride spray in South Korea on March 21. The company secured the exclusive rights for Finjuve Spray from Almirall (Spain) in January 2022.
Note: I am even more interested in topical Dutasteride for hair loss.
Will Topical Finasteride have Fewer Side Effects?
Due to some people getting persistent side effects from oral finasteride, the interest in topical finasteride is extremely high. While there are no guarantees when it comes to fewer side effects from topical finasteride, it is a virtual certainty that there will not be higher rates of side effects.
Speaking for myself, it does give me peace of mind to use something topically rather than ingesting something orally. Moreover, with the topical product, one can use a lot less than the recommended dosage. If you are only losing hair in the crown region, you might start by applying a bare minimum of the spray or lotion in that area.
This is a lot more desirable than ingesting a whole pill. Breaking tiny 1 mg finasteride (Propecia) pills into even smaller pieces and trying to distribute the resulting dosage evenly is not practical. Make sure to also read my post on finasteride and dutasteride dosage discussion.
Note that Hasson & Wong in Canada has been prescribing its own topical finasteride for a number of years, but they have not had to undertake any clinical trials. They use a local compounding pharmacy as well as Farmacia Parati (Italy) to make their product. In the past, they told me that the topical product has fewer side effects than the oral product.
A number of other hair transplant clinics nowadays offer proprietary combination topical products that contain both finasteride and minoxidil. Some doctors prefer oral finasteride to topical for generalized hair loss.
Serum, Plasma and Scalp DHT Level Reduction
Research on dihydrotestosterone (DHT) level reduction by topical finasteride shows varying results. This is obviously impacted by the type of compounded product being used, dosage amount and application frequency.
One study of P-3074 found scalp DHT levels declined by 70% with once a day application of 1 ml (2.275 mg) of P-3074 topical finasteride. By comparison, oral finasteride 0.5 mg/day reduces scalp DHT levels by around 50-70 percent (different results in different studies). However, the P-3074 study showed 50 percent scalp DHT reduction when using lower doses.
Serum DHT was reduced by 60 -70 % for the 1 ml dose of P-3074. Similar to what is seen with oral finasteride. But only 25 percent with the 100 and 200 μL doses; and around 45 percent with the 300 and 400 μL doses.
Interestingly, no relevant changes occurred in serum testosterone readings. By contrast, oral finasteride changes both testosterone and estrogen levels.
Almirall will release ALM12845 in Europe in 2021
A few months ago, I contacted Almirall and they told me that they would release their topical finasteride ALM12845 (or ALM-12845) in Western Europe in 2021. The release date would vary by country. No plans for selling in the US market.
One of this blog’s readers (“Paul”) e-mailed them after me, and he was given the more specific release date statement below:
“Without going beyond the limits of confidentiality, I can confirm you that we expect to launch topical Finasteride by the beginning of 2021 in Europe once the assessment by Health authorities is completed.”
Earlier today, reader “Lesley” made an important discovery. She posted this recent link from Gazzeta Ufficiale (Italy). It has the English translation of part of the content from this pdf. I will discuss the content in more detail further below.
Difa Cooper’s Caretopic Topical Finasteride
Per the above news, it seems like Italy based Difa Cooper (also known as Cantabria Labs Difa Cooper) will produce Almirall’s ALM12845. Per “Lesley”, they will only market it in France and Germany. I cannot tell for sure, but makes sense per below image.
In January 2020, Almirall announced that ALM12845 no longer fits its strategic portfolio. At the time, the company had started looking for a partner to out-license the product. The first partner is now likely Difa Cooper. I am guessing that they will need more partners in other European countries.
Per the earlier mentioned Gazzeta link:
- The topical finasteride (brand name “Caretopic“) will be in “cutaneous spray, solution” form.
- Each ml of the solution will contain 2.275 mg of finasteride. Each bottle will contain 18 ml.
- Each dispersion (spray) amounts to 50 μl, which contains 114 micrograms of finasteride.

Finjuve
In November 2021, Almirall and Hikma Pharmaceutical entered into an exclusive licensing agreement. This will allow Himka to commercialize and market Almirall’s topical finasteride via the FinjuveTM brand in certain Middle East and North Africa (MENA) markets. In January 2022, Boryung Pharmaceutical signed a contract with Almirall to sell Finjuve spray-type topical finasteride in South Korea.
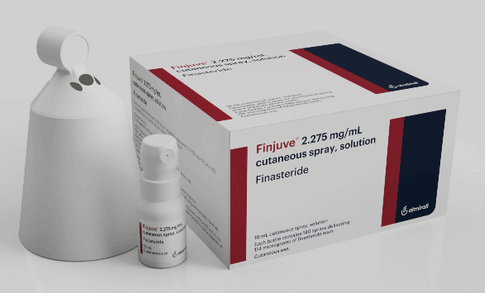
Finjuve is a finasteride spray that is approved in Italy, Germany, Luxembourg and Portugal for the treatment of androgenetic alopecia. Interestingly, Finjuve is meant to be applied to the scalp through a cone. This prevents the spray produce from spreading outside the treatment area, and improves overall safety.
Almirall CEO Interview
There is a new November 2021 interview of Almirall CEO Gianfranco Nazzi. In regards to their topical Finasteride, Mr. Nazzi states as follows (slightly modified quote):
“It is a legacy Polichem product. We have not launched it, but are licensing it out. We do see some small incoming milestone income for this topical Finasteride from Eastern Europe and other countries within Europe. But is not going to be a significant mover of the company. We’re just trying to maximize the value we can get out of it.”